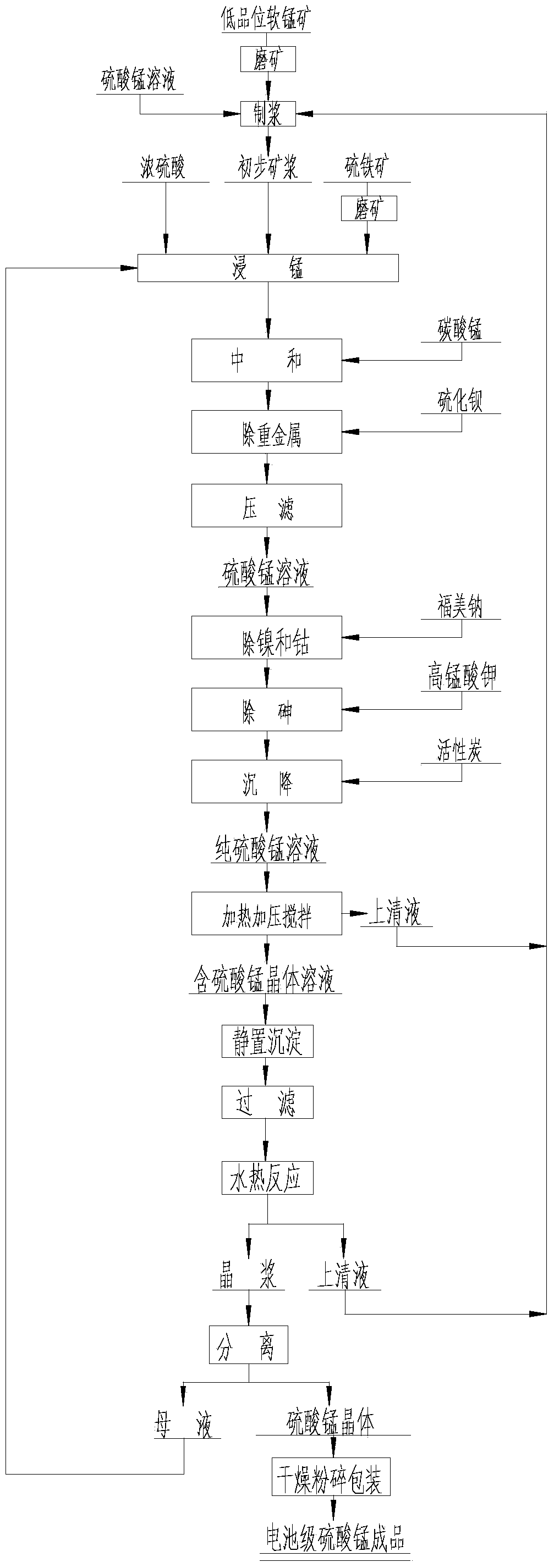
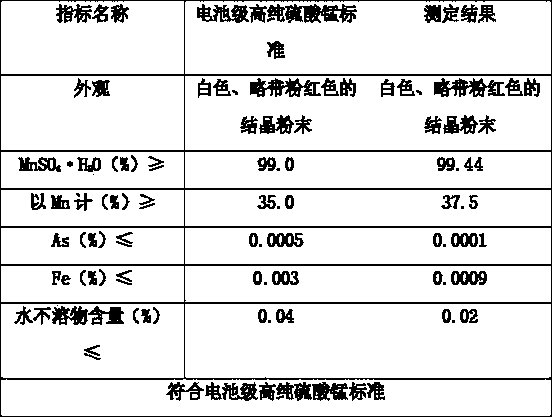
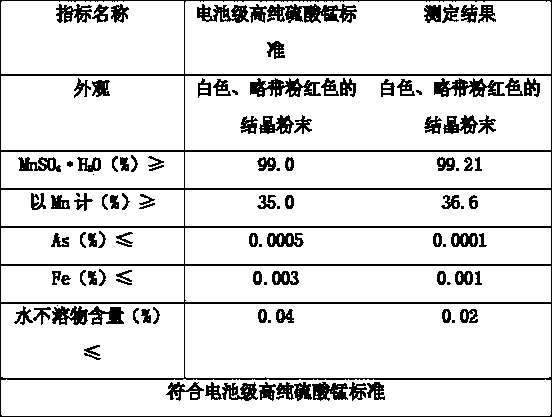
Method for preparing cell-grade high-purity manganese sulfate by low-grade manganese ore high-pressure crystallization
A low-grade manganese ore and high-pressure crystallization technology, applied in the direction of manganese sulfate, etc., can solve the problems of large equipment corrosion, high energy consumption, and large sulfuric acid consumption, and achieve high profits, increase production, and reduce environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]The low-grade pyrolusite and pyrite are respectively ball-milled into powders with a particle size of 0.01mm. Grinding is one of the most important links in the leaching process. If the particle size is too small, the cost of grinding will increase. The leaching rate of manganese is greatly reduced, which directly affects the final production cost; the manganese sulfate solution with a concentration of 60g / L is mixed with pyrolusite powder to obtain preliminary pulp, and 100kg of preliminary pulp and 10kg of 0.01mm particle size are obtained. Pyrite powder and 20kg of concentrated sulfuric acid are prepared according to the mass ratio of the initial pulp: concentrated sulfuric acid: pyrite = 1:0.2:0.1. The temperature is heated to 75°C and kept at this temperature for 180min of manganese leaching. Such ratio, manganese leaching time and heating temperature can obtain good leaching results; The pH value of the mixed pulp is 6.5, so that the ferric ions in the pulp are pre...
Embodiment 2
[0034] The low-grade pyrolusite and pyrite were ball-milled into powders with a particle size of 0.03 mm, respectively, and a manganese sulfate solution with a concentration of 70 g / L was mixed with the pyrolusite powders to obtain a preliminary pulp. 0.03mm pyrite powder and 25kg of concentrated sulfuric acid are prepared according to the mass ratio of the initial pulp: concentrated sulfuric acid:pyrite=1:0.25:0.13 ratio, put the prepared material into the manganese soaking bucket with stirring, The temperature in the barrel was heated to 80°C and kept at this temperature for 180 min of manganese leaching, and the neutralizing agent manganese carbonate ore powder was added to the pulp to adjust the pH value. The pH value of the neutralized pulp was 6.1, so that the ferric ions in the pulp were Precipitate in the form of ferric hydroxide, so as to achieve the purpose of removing iron, add barium sulfide to the neutralized manganese sulfate-containing ore slurry, and perform pre...
Embodiment 3
[0038] The low-grade pyrolusite and pyrite were ball-milled into powders with a particle size of 0.05mm, and a manganese sulfate solution with a concentration of 80g / L was mixed with the pyrolusite powders to obtain a preliminary pulp. 0.05mm pyrite powder and 30kg concentrated sulfuric acid are prepared according to the mass ratio of the initial pulp: concentrated sulfuric acid:pyrite=1:0.3:0.15 ratio, put the prepared material into the manganese leaching bucket with stirring, The temperature in the barrel was heated to 85°C and kept at this temperature for 180min of manganese leaching, and the neutralizing agent manganese carbonate ore powder was added to the pulp to adjust the pH value. The pH value of the neutralized pulp was 5.8, so that the ferric ions in the pulp were Precipitate in the form of ferric hydroxide, so as to achieve the purpose of removing iron, add barium sulfide to the neutralized manganese sulfate-containing ore slurry, and perform pressure filtration aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com