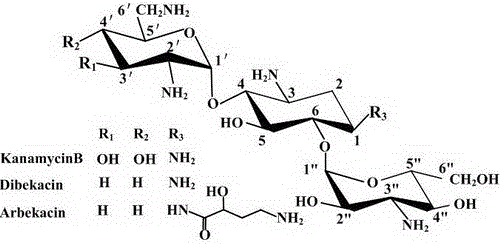
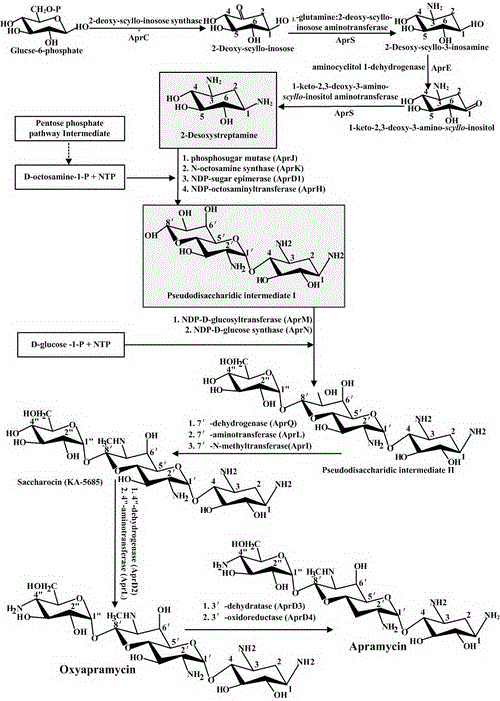
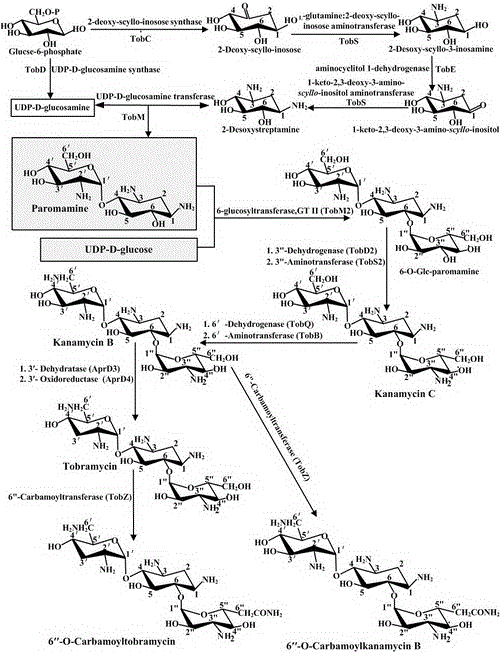
Engineering bacteria producing kanamycin b and its construction and application
A technology for producing kanamycin and kanamycin, which is applied to bacteria, biochemical equipment and methods, and the introduction of foreign genetic material using vectors, etc., can solve the problems of not making breakthroughs and restraining the genetic modification of Streptomyces kanamycin, etc. Achieve the effect of single component, omit alkaline high temperature hydrolysis process, and eliminate pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: Construction of recombinant plasmid pBD5
[0045] Using S. tenebrariusTt-49 chromosomal DNA as a template, use primers PD1 / PD2 to amplify about 2000bp of the upstream exchange arm BD1, which includes the partial sequence of aprD3-D4 and its upstream fragment. The PCR product is digested with EcoRI and XbaI and ligated to The intermediate plasmid pBD3 was obtained on the PKC1139 vector digested with the same enzyme. Then, using Tt-49 chromosomal DNA as a template, primers PD3 / PD4 are used to amplify the downstream exchange arm BD2 of about 2000bp, which includes aprD3-D4 partial sequence and its downstream fragment, and the PCR product is digested with XbaI and HindIII and ligated to the On the pBD3 digested with the same enzyme, the intermediate plasmid pBD4 was obtained. Finally, the plasmid pAGe containing the erythromycin resistance gene was digested with EcoRI, and the 1746bp fragment was recovered and connected to pBD4 that had been digested with Eco...
Embodiment 2
[0051] Example 2: Transformation of S.tenebrariusTt-49 with the recombinant plasmid pBD5
[0052] Transform the recombinant plasmid pBD5 into E.coliET12567 (pUZ8002) to obtain the donor strain E.coliET12567 (pUZ8002, pBD5) containing the recombinant plasmid. After overnight culture, transfer to 30ml and add corresponding antibiotics (kanamycin 25μg / ml, chloramphenicol 25 μg / ml apramycin, 50 μg / ml apramycin) in LB medium, cultured for 2-3 hours to make the bacteria enter the logarithmic growth phase, collected the bacteria by centrifugation at 8000 rpm for 5 minutes, washed twice with an equal volume of fresh LB to remove residual Antibiotics, suspended in an appropriate amount of LB medium for later use. At the same time, scrape an appropriate amount of mature and plump S. tenebrariusTt-49 slant spores and suspend them in 2×YT medium, heat shock at 50°C for 10 minutes, and cool to room temperature. Mix the spore suspension and Escherichia coli suspension in equal proportions,...
Embodiment 3
[0053] Example 3: Screening of Knockout aprD3-D4 Gene Mutants
[0054] Transfer the single-crossover mutant strain to the slant medium, isolate a single colony after 5 generations of relaxation culture, and copy it to the resistance plate added with erythromycin and the ordinary plate without antibiotics. After culturing, 7 strains were screened and grown on the ordinary plate The erythromycin-sensitive type (Ery S ) strains. These Ery S The strain may be an aprD3-D4 blocking mutant strain, or a reverting mutant strain. In order to finally screen out the aprD3-D4 blocking mutant strain, screening and verification is carried out by PCR method.
[0055] D2 strain was selected, chromosomal DNA was extracted, and primers PD5 / PD6 and PD7 / PD8 were designed for PCR verification analysis of D2. Using PD5 / PD6 primers for PCR, a 2452bp band can be amplified, and a 6701bp band can be amplified theoretically using PD7 / PD8, but in fact the target cannot be amplified because the amplific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com