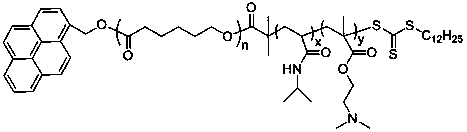
Preparation method of temperature and CO2 double-responsive block copolymer nano micelle
A block copolymer and nanomicelle technology, which is applied in the fields of polymer materials and biomedical engineering, can solve the problems of lack of strong hydrophobicity, high crystallinity, and no expected biological reactivity of cells or tissues. The effect of the simple and easy synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]Take 0.50 g of pyrene methanol, 10.00 g of caprolactone, and 87.7 μL of stannous octoate into a round-bottomed flask, vacuumize and fill with nitrogen three times, and react with magnetic stirring at 120°C for 24 hours. After the reaction, dissolve the product in distilled water Chloromethane, precipitated with methanol, and vacuum-dried to obtain the polymer Py-PCL-OH. Py-PCL-OH 7g, 2-(dodecyltrithiocarbonate)-2-methylpropionic acid 5.1g, N,N-dicyclohexylcarbodiimide (DCC) 2.887g, 4- Add 0.941g of dimethylaminopyridine (DMAP) and 20mL of N,N-dimethylformamide into the reactor, vacuumize and fill with nitrogen three times, under the protection of argon, stir the reaction at room temperature for 24 hours with magnetic force. After desalting by suction filtration, precipitation with deionized water, and vacuum drying, the RAFT macromolecular chain transfer agent whose main chain is PCL was obtained. Weigh 1 g of RAFT macromolecular chain transfer agent and dissolve in 6 m...
Embodiment 2
[0026] Take 0.445g of pyrenemethanol, 8.9g of caprolactone, and 78μL of stannous octoate into a round-bottomed flask, vacuumize and fill with nitrogen three times, and react with magnetic stirring at 120°C for 24h. After the reaction, dissolve the product in trichloro methane, precipitated with n-hexane, and vacuum-dried to obtain the polymer Py-PCL-OH. Py-PCL-OH 7g, 2-(dodecyltrithiocarbonate)-2-methylpropionic acid 5.1g, N,N-dicyclohexylcarbodiimide (DCC) 2.887g, 4- Add 0.941 g of dimethylaminopyridine (DMAP) and 20 mL of dichloromethane into the reactor, vacuumize and fill with nitrogen three times, and react with magnetic stirring at room temperature for 24 hours under the protection of argon. After desalting by suction filtration, precipitation with deionized water, and vacuum drying, the RAFT macromolecular chain transfer agent whose main chain is PCL was obtained. Weigh 1 g of RAFT macromolecular chain transfer agent and dissolve in 8 mL of anisole, add 2.836 g of N-is...
Embodiment 3
[0028] Take 0.50 g of pyrene methanol, 10.00 g of caprolactone, and 87.7 μL of stannous octoate into a round-bottomed flask, vacuumize and fill with nitrogen three times, and react with magnetic stirring at 120°C for 24 hours. After the reaction, dissolve the product in three Chloromethane, precipitated with methanol, and vacuum-dried to obtain the polymer Py-PCL-OH. Py-PCL-OH 7g, 2-(dodecyltrithiocarbonate)-2-methylpropionic acid 5.1g, N,N-dicyclohexylcarbodiimide (DCC) 2.887g, 4- Add 0.941 g of dimethylaminopyridine (DMAP) and 20 mL of dichloromethane into the reactor, vacuumize and fill with nitrogen three times, and react with magnetic stirring at room temperature for 24 hours under the protection of argon. After desalting by suction filtration, precipitation with deionized water, and vacuum drying, the RAFT macromolecular chain transfer agent whose main chain is PCL was obtained. Weigh 1 g of RAFT macromolecular chain transfer agent and dissolve in 6 mL of dioxane, add 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com