Mutant TBA-H2 of acid and high temperature resistant beta-amylase and application thereof
A technology of TBA-H2 and amylase, applied in the direction of enzyme, hydrolase, glycosylase, etc., can solve the problem that β-amylase cannot take into account acid resistance and temperature resistance at the same time, so as to improve catalytic activity and wide application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
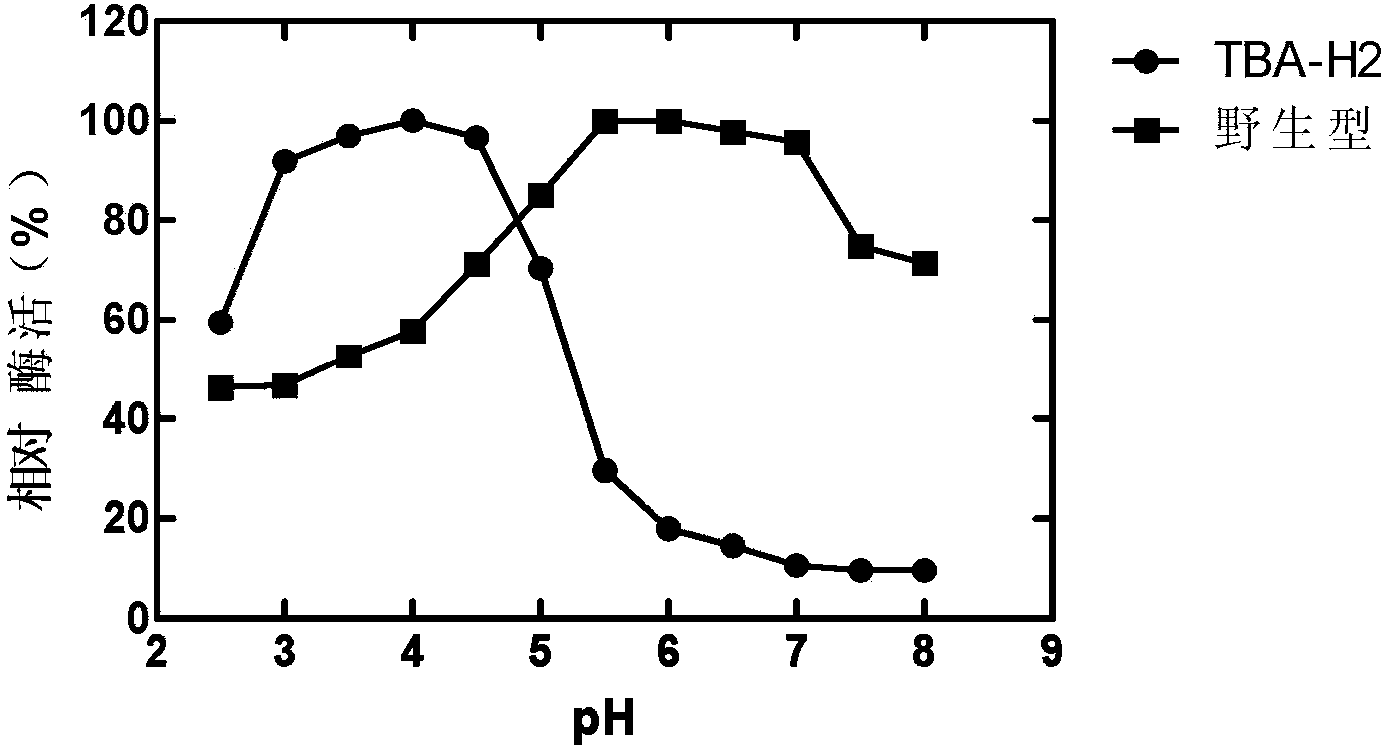
[0017] This example illustrates the method for obtaining the β-amylase mutant TBA-H2.
[0018] 1) Cloning of β-amylase gene ctba
[0019] Using Thermoanaerobacterium thermosulfurigenes (Thermoanaerobacterium thermosulfurigenes, American Type Culture Collection No. ATCC33743) chromosomal DNA as template, SEQ ID NO: 2 as upstream primer (containing a Nco I restriction site) and SEQ ID NO: 3 (contains a Bam HI restriction site and introduces a 6x histidine tag coding sequence) as a downstream primer to amplify the mature peptide coding region of β-amylase by polymerase chain reaction (PCR), corresponding to the ctba gene The 660-2219 fragment in the center, the length of the obtained target product is 1605bp; the target product and the pSE380 vector plasmid were double-digested with Nco I and Bam HI enzymes respectively, after the gel was recovered, they were ligated with T4 DNA ligase, and transformed into Escherichia coli (E. .coli) XL1-Blue competent cells; select transforman...
Embodiment 2
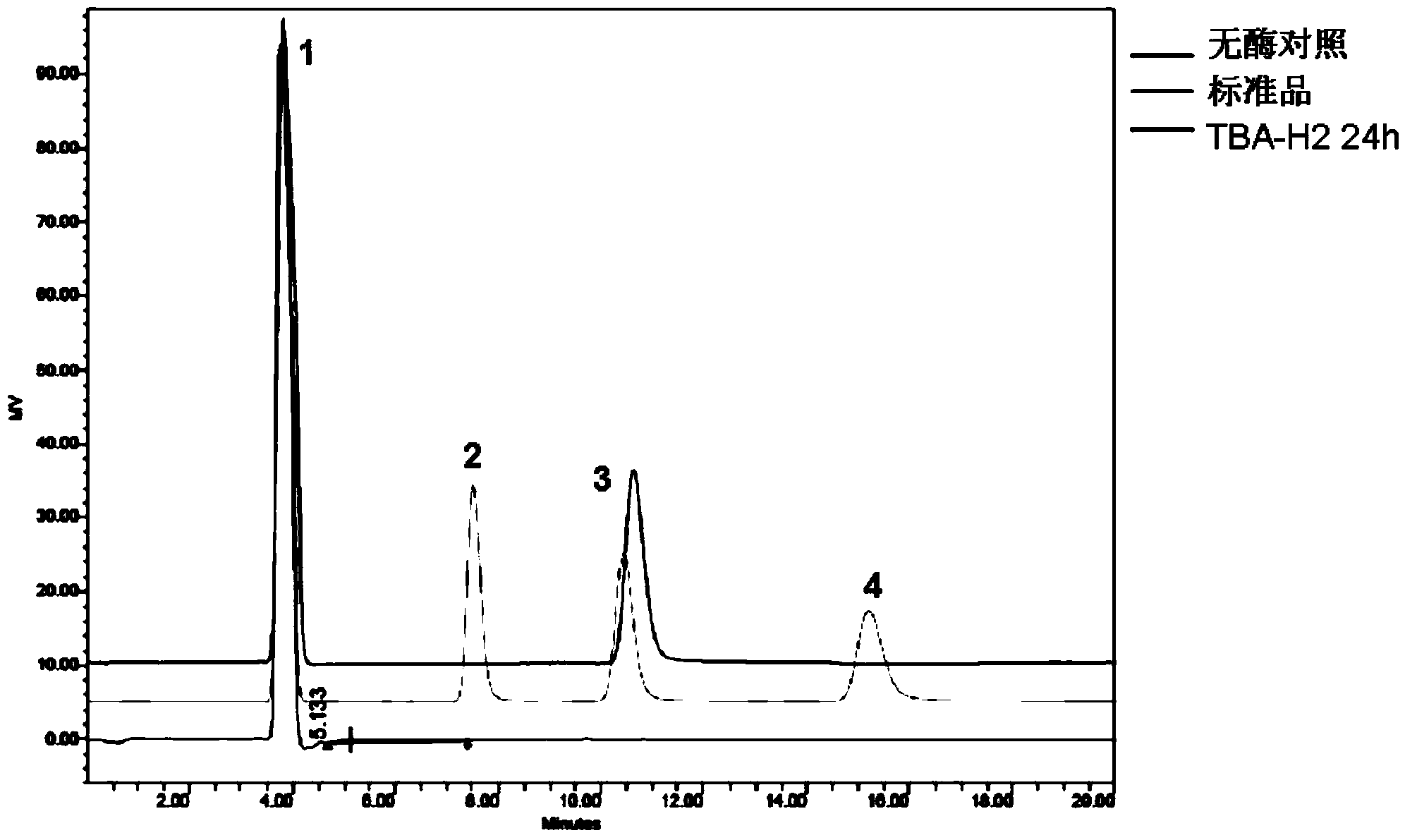
[0026] This example illustrates the application of mutant enzyme TBA-H2 in the production of high-purity maltose syrup by hydrolyzing starchy raw materials
[0027] Take 1g of potato soluble starch and add 0.05mol / L pH4.0 acetic acid buffer solution to prepare 1% (mass percentage) starch slurry, add purified enzyme solution according to the amount of 1.2mg enzyme / g dry matter starch, and then place it in 60 Warm in a water bath at ℃ for 24 hours, heat treatment in a water bath at 100 ℃ for 10 minutes, centrifuge at 12,000 r / min for 5 minutes, filter the supernatant through a 0.22 μm filter membrane, and take 20 μL of the filtrate to pass through an amino column for high performance liquid chromatography (HPLC) detection of the enzymatic hydrolysis product . The detection conditions of the amino column were RI temperature of 50°C, flow rate of 1mL / min, and mobile phase of acetonitrile:water (70:30). Amino column detection results such as image 3 shown. It can be seen that u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com