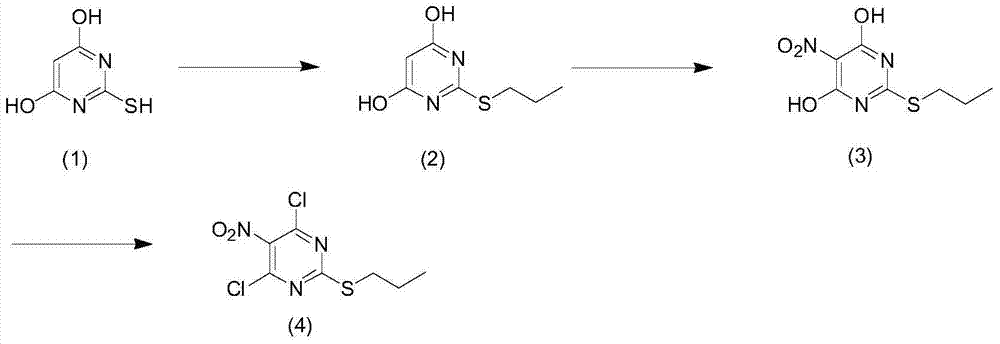
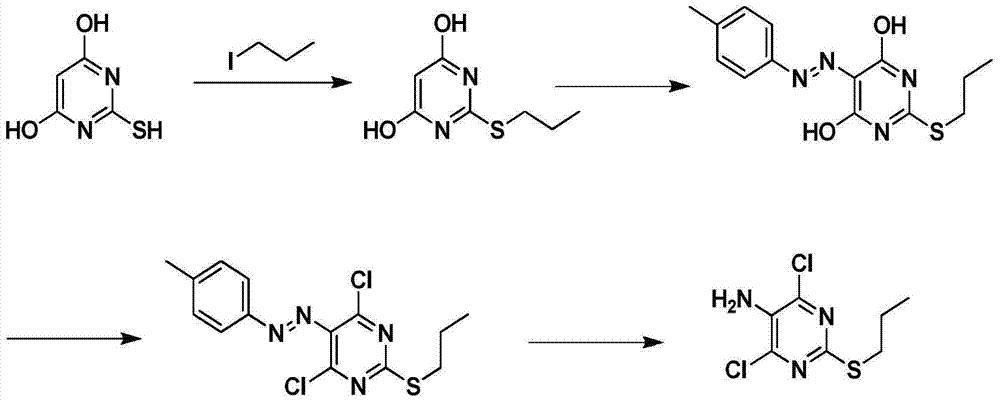
Preparation method of 2-propylthio-4,6-dichloro-5-aminopyrimidine
An aminopyrimidine and propylthio technology, which is applied in the field of preparation of 2-propylthio-4,6-dichloro-5-aminopyrimidine, can solve the high pressure of hydrogenation reduction of azo compounds and increase the industrial production cost of cargreol , the problem of expensive starting materials, etc., to achieve the effect of reducing production costs, developing a large market, and improving productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
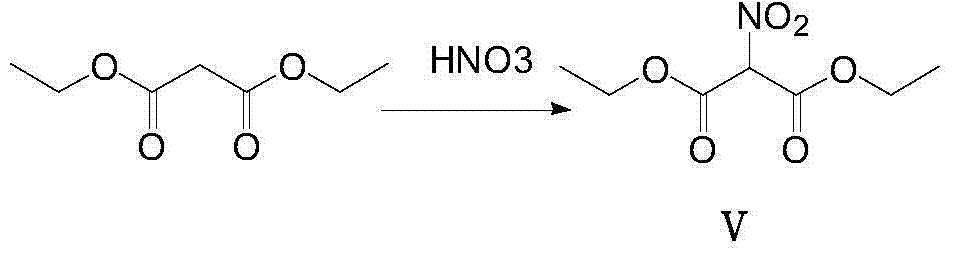
[0038] (1) Synthesis of Compound V
[0039]
[0040] Weigh 20g of diethyl malonate and put it into a 250ml round bottom flask, slowly add 20ml of fuming nitric acid dropwise under ice bath, and control the temperature below 15°C. After stirring at room temperature for 2 hours, the mixture was poured into ice water, then extracted twice with dichloromethane, and the organic layers were combined; the organic layers were washed with water and 5% aqueous urea solution until the starch potassium iodide test paper did not develop color. The dichloromethane solution was extracted with 10% sodium carbonate solution, the combined aqueous layers were acidified to pH ~ 4 with concentrated hydrochloric acid, then extracted with dichloromethane, the combined extracts were dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation. 24g of product compound V was obtained, yield: 93.75%. 1 H-NMR (400MHz, CDCl3) δ: 5.83(s, 1H), 4.46(q, J=4.0Hz, 4H), 1....
Embodiment 2
[0054] (1) Synthesis of compound V
[0055]
[0056] Take 20g of diethyl malonate and put it into a 250ml round bottom flask, slowly add 5ml of fuming nitric acid dropwise under ice bath, and control the temperature below 15°C. Stir at room temperature for 2 hours, pour the mixture into ice water, then extract twice with dichloromethane, and combine the organic layers; the organic layers were washed with water and 5% urea aqueous solution, respectively, until the starch potassium iodide test paper did not develop color. The dichloromethane solution was extracted with 10% sodium carbonate solution, the combined aqueous layers were acidified to pH 2 with concentrated hydrochloric acid, then extracted with dichloromethane, the combined extracts were dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation to obtain 24g Product compound V, yield: 93.75%. 1 H-NMR (400MHz, CDCl3) δ: 5.83(s, 1H), 4.46(q, J=4.0Hz, 4H), 1.38(t, J=4.0Hz, 6H). ...
Embodiment 3
[0070] (1) Synthesis of compound V
[0071]
[0072] Weigh 20g of diethyl malonate and put it into a 250ml round bottom flask, slowly add 80ml of fuming nitric acid dropwise under ice bath, and control the temperature below 15°C. After stirring at room temperature for 2 hours, the mixture was poured into ice water, then extracted twice with dichloromethane, and the organic layers were combined; the organic layers were washed with water and 5% aqueous urea solution until the starch potassium iodide test paper did not develop color. The dichloromethane solution was extracted with 10% sodium carbonate solution, the combined aqueous layers were acidified to pH 2 with concentrated hydrochloric acid, then extracted with dichloromethane, the combined extracts were dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation to obtain 24g Product compound V, yield: 93.75%. 1 H-NMR (400MHz, CDCl3) δ: 5.83(s, 1H), 4.46(q, J=4.0Hz, 4H), 1.38(t, J=4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com