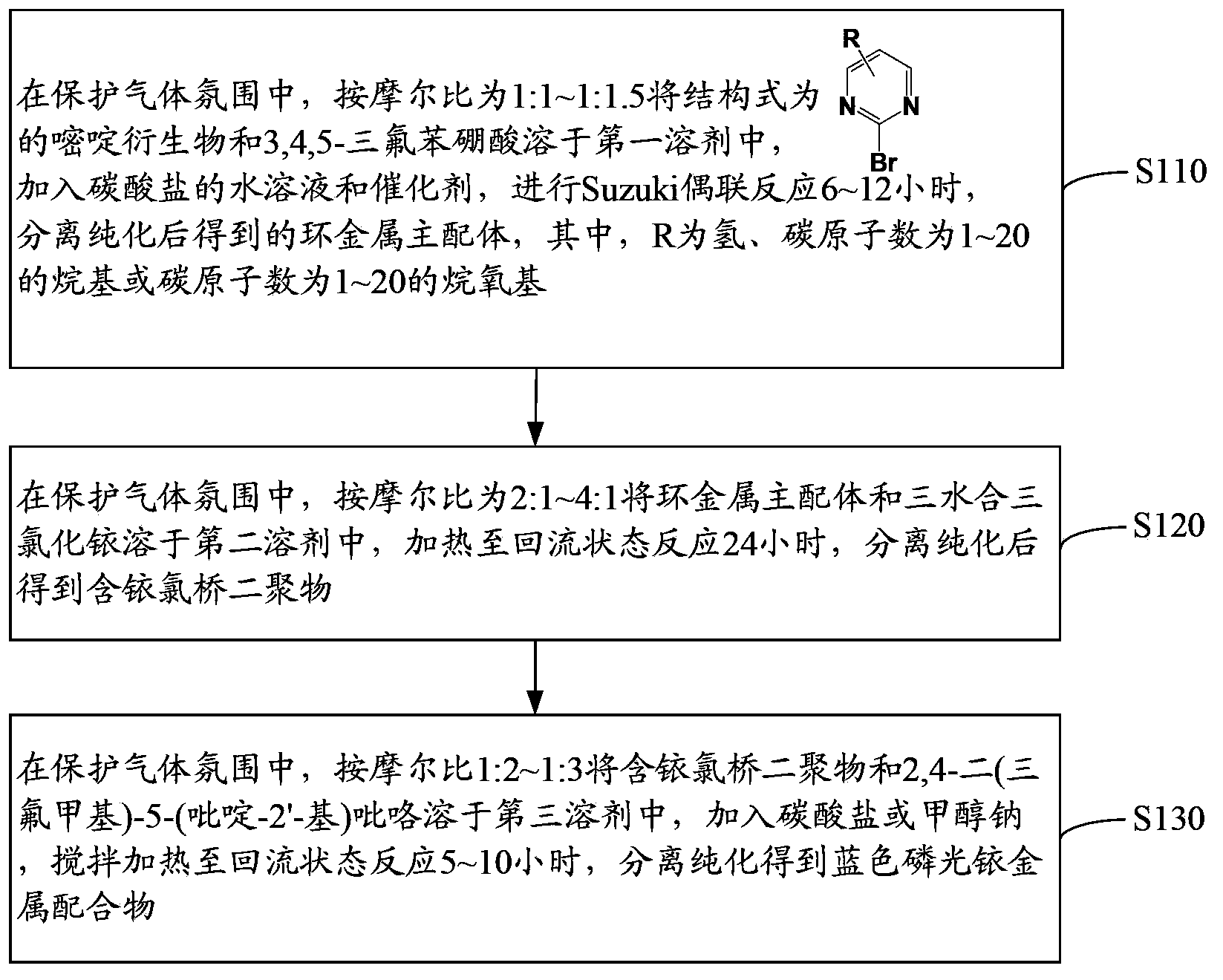
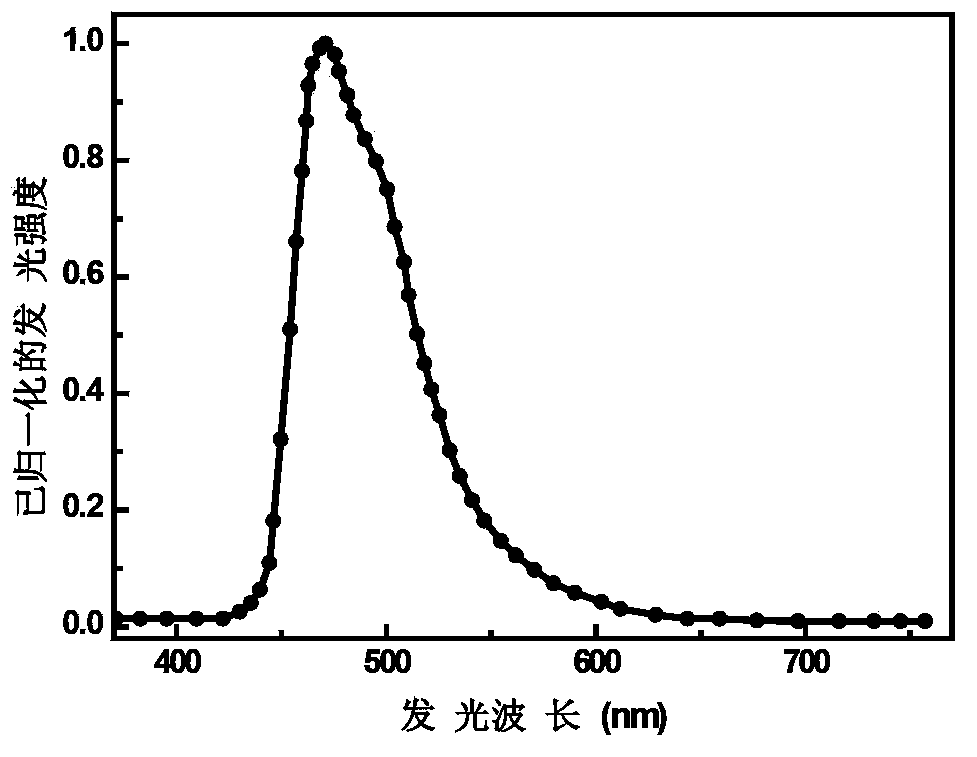
Blue phosphorescent iridium metal complex, preparation method thereof and organic electroluminescent device
A technology of iridium metal complexes and blue phosphorescence, which is applied in the fields of electric solid-state devices, organic chemistry, and light-emitting materials, can solve the problems of poor light-emitting performance and development lag of light-emitting devices, and achieve improved light-emitting performance, reduced direct effects, and improved The effect of luminous properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] The preparation method of the blue phosphorescent iridium metal complex has mild reaction conditions, low equipment requirements, low preparation cost and easy large-scale preparation.
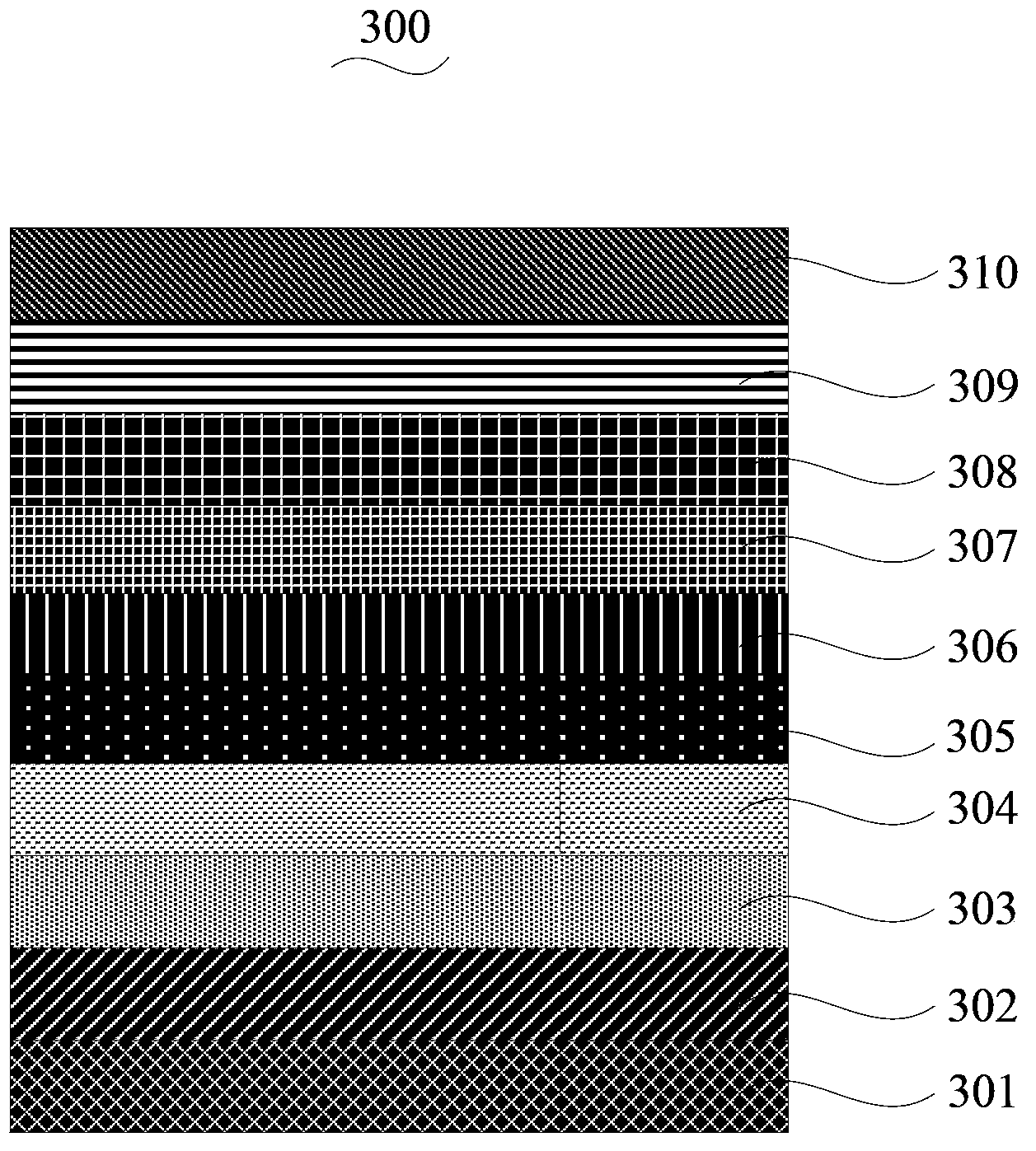
[0072] see figure 2 , an organic electroluminescent device 300 according to one embodiment, including a substrate 301, an anode 302, a hole injection layer 303, a hole transport layer 304, an electron blocking layer 305, a light emitting layer 306, a hole blocking layer 307, and an electron layer stacked in sequence. Transport layer 308 , electron injection buffer layer 309 and cathode 310 .
[0073] The materials of the substrate 301, the anode 302, the hole injection layer 303, the hole transport layer 304, the electron blocking layer 305, the hole blocking layer 307, the electron transport layer 308, the electron injection buffer layer 309 and the cathode 310 are commonly used in the art. Material. For example, the substrate 101 is a glass substrate, the material of the anode 302 ...
Embodiment 1
[0078] Blue phosphorescent bis(2-(3',4',5'-trifluorophenyl)pyrimidine-N,C 2 ') Synthesis of (2,4-bis(trifluoromethyl)-5-(pyridin-2'-yl)pyrrole)iridium complexes.
[0079] Blue phosphorescent bis(2-(3',4',5'-trifluorophenyl)pyrimidine-N,C 2 ')(2,4-bis(trifluoromethyl)-5-(pyridine-2'-yl)pyrrole) iridium complex has the following structural formula:
[0080]
[0081] (1) Synthesis of 2-(3',4',5'-trifluorophenyl)pyrimidine
[0082] In a nitrogen atmosphere, 1.59 g (10 mmol) of 2-bromopyrimidine, 2.11 g (12 mmol) of 3,4,5-trifluorophenylboronic acid and 0.58 g (0.5 mmol) of tetrakis(triphenylphosphine)palladium were dissolved in 40 mL In toluene, then 20 mL of an aqueous solution containing 2.76 g (20 mmol) of potassium carbonate was added dropwise to the reaction system. Heated and stirred at 100°C for 6h. After the reaction was completed, after the reaction solution was cooled to room temperature, extracted with dichloromethane, the organic phase was taken, washed with water...
Embodiment 2
[0108] Blue phosphorescent bis(2-(3',4',5'-trifluorophenyl)-5-methylpyrimidine-N,C 2 ') Synthesis of (2,4-bis(trifluoromethyl)-5-(pyridin-2'-yl)pyrrole)iridium complexes.
[0109] Blue phosphorescent bis(2-(3',4',5'-trifluorophenyl)-5-methylpyrimidine-N,C 2 ')(2,4-bis(trifluoromethyl)-5-(pyridine-2'-yl)pyrrole) iridium complex has the following structural formula:
[0110]
[0111] (1) Synthesis of 2-(3',4',5'-trifluorophenyl)-5-methylpyrimidine
[0112] In an argon atmosphere, 1.73g (10mmol) of 2-bromo-5-methylpyrimidine, 1.76g (10mmol) of 3,4,5-trifluorophenylboronic acid and 0.28g (0.4mmol) of dichlorobis(triphenyl Phosphorus) palladium was dissolved in 50 mL of DMF, and then 25 mL of an aqueous solution containing 3.18 g (30 mmol) of sodium carbonate was added dropwise to the reaction system. Stir the reaction under heating to 90°C for 8 hours. After the reaction was completed, after the reaction solution was cooled to room temperature, it was extracted with dichlor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Maximum lumen efficiency | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com