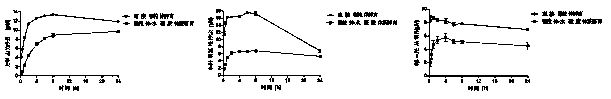Metabolic enzyme-hydrogel system for drug metabolism, drug effect and toxicity evaluation
A hydrogel and metabolic enzyme technology, applied in the field of metabolic enzyme-hydrogel system, to achieve accurate evaluation of drug efficacy or toxicity, no effect on cell viability, and suitable spatial structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Construction of metabolic enzyme-hydrogel system
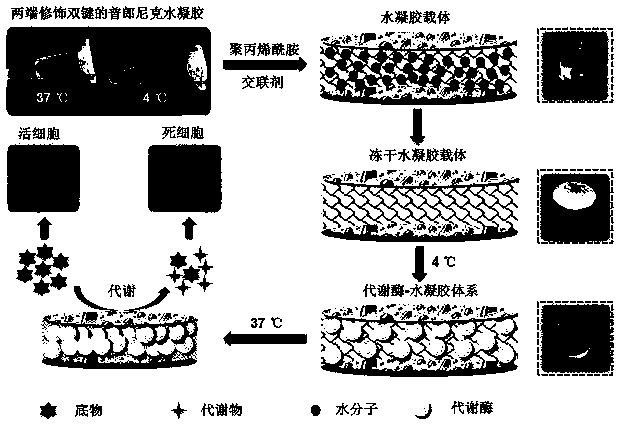
[0028] Preparation steps such as figure 1 as shown,
[0029] (1) Through the condensation reaction of acryloyl chloride and F127 under alkaline conditions, F127 was successfully modified with ester bonds to have double bonds at both ends; the macromonomer was dissolved in deionized water to prepare 30% Concentration mother solution, stored at 4°C;
[0030] (2) To prepare 100 ul hydrogel carrier, absorb 33 ul double bond F127 mother solution, 1 ul purchased 30% acrylamide:bisacrylamide (29:1) mixed solution, 1 ul 10% ammonium persulfate solution, 0.5 ul TEMED, and the rest with 64.5 ul PBS phosphate buffer solution; transfer the mixture to a 96-well plate, carry out cross-linking at room temperature or 37°C, and obtain a hydrogel after about 30 min;
[0031] (3) Place the hydrogel obtained in the above step (2) into 5% mannitol solution and shake and wash it repeatedly, about 5 times, 20 min each time;
...
Embodiment 2
[0034] Example 2 Metabolism of microsome-hydrogel system
[0035] Such as figure 2 As shown, microsomes contain almost all of a metabolic enzyme, and a metabolic enzyme is responsible for the biotransformation process of most drugs. Therefore, several representative metabolic enzymes including CYP3A, CYP2D6, and CYP2E1 are selected for verification Metabolic capacity of microsome-hydrogel systems.
[0036]The standard substrates of the three enzymes certified by the FDA, 90μM testosterone (CYP3A), 100μM dextromethorphan (CYP2D6) and 200μM chlorzoxazone (CYP2E1), were used for incubation at 37°C; in the 500ul incubation system, 50ul of the above concentration Substrate, 100ul metabolic reaction initiation system NADPH (final concentration 1mM), 350ul PBS buffer and 100ul microsome-hydrogel; 50 ul samples were drawn at 6 h, 8 h and 24 h, and the content of the corresponding substrates 6β-OH testosterone, nordextromethorphan and 6-OH testosterone in the sample was analyzed b...
Embodiment 3
[0037] Example 3 Drug efficacy and toxicity prediction ability of microsome-hydrogel system
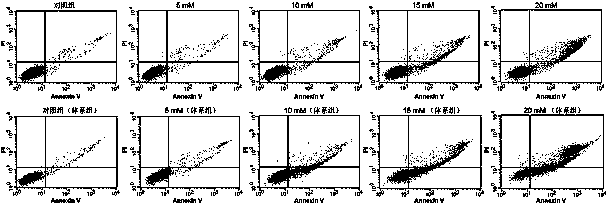
[0038] Such as image 3 As shown, cyclophosphamide is a classic anti-tumor prodrug, which can be transformed into active nitrogen mustard only after being metabolized in the body, so as to exert its anti-tumor ability. Cyclophosphamide was selected as the model drug, and human breast cancer MCF-7 cells were used as the test cells to investigate the influence of the presence or absence of the microsome-hydrogel system on its antitumor efficacy.
[0039] MCF-7 cells were treated with 5 mM, 10 mM, 15 mM and 20 mM cyclophosphamide for 24 hours, and the microsome-hydrogel system was added to one group of the culture system, and the control group without the system was set up, and the collected Cells were stained with Annexin V / PI, and finally the degree of apoptosis was analyzed by flow cytometry; the results were as follows image 3 As shown, the number of apoptotic cells added to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com