A kind of preparation method of furazolidone preparation
A technology of furazolidone and preparation, applied in the field of medicine, can solve problems such as limitation, and achieve the effects of fast drug release, improved therapeutic effect and high drug release amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
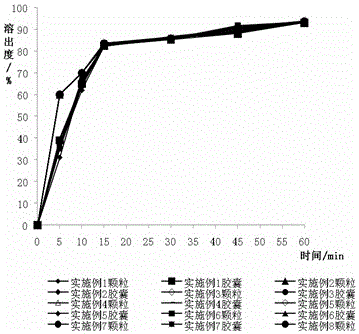
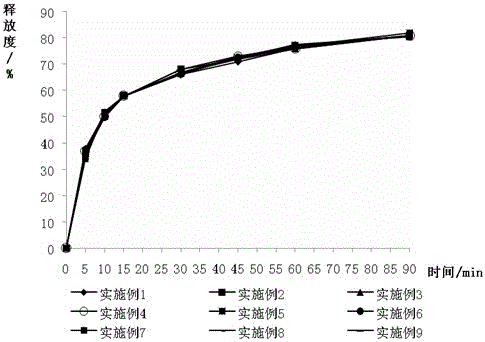
Image
Examples
Embodiment
[0081] Take 100 g of furazolidone and 10 g of starch and mix evenly, add 0.4 g of sodium lauryl sulfate and 0.8 g of caprylic acid macrogolglyceride to make a soft material made of povidone aqueous solution with a concentration of 20%, and the amount of povidone is 6 g . Granulate with 16 mesh, dry at 70°C, granulate with 20 mesh, add 1.5g magnesium stearate, 1.0g silicon dioxide and 4g sodium carboxymethyl starch, mix evenly, and fill enteric-coated capsules.
[0082] Example: 2
[0083] Take 100 g of furazolidone and 4 g of pregelatinized starch and mix evenly, add 10% mass concentration of povidone aqueous solution containing 0.5 g of poloxamer and 0.5 g of diethylene glycol monoethyl ether to make a soft material, and the amount of povidone is 20 g . Granulate with 18 mesh, dry at 60°C, granulate with 16 mesh, add 0.5g of magnesium stearate, 0.5g of silicon dioxide and 2g of sodium carboxymethyl starch, mix evenly, and fill enteric-coated capsules.
[0084] Example: 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com