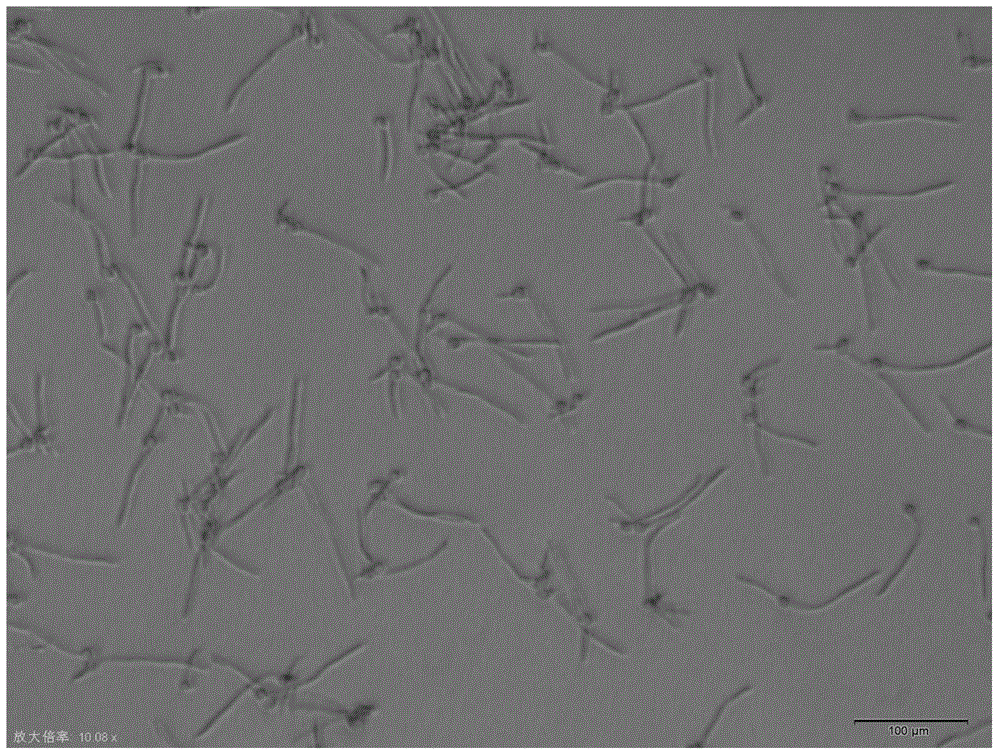
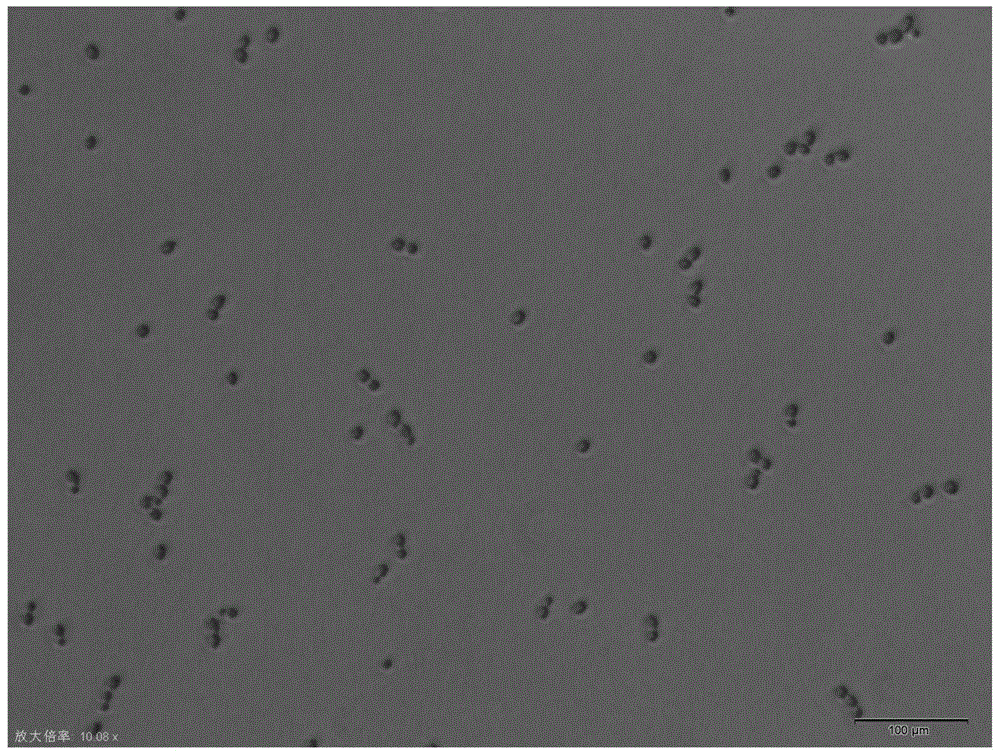
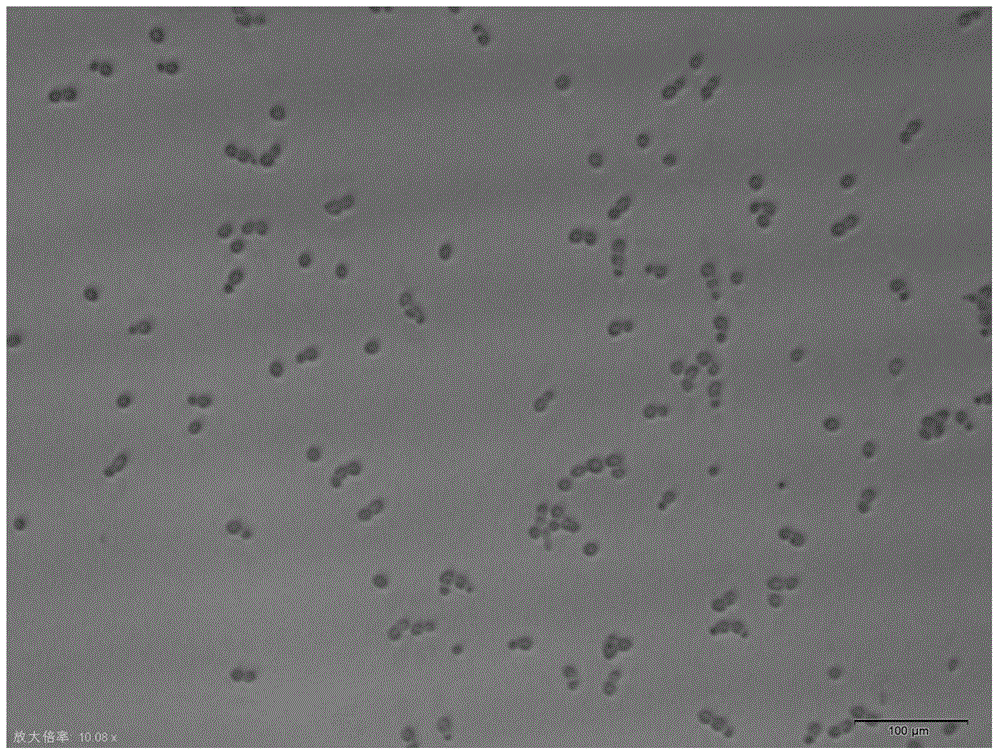
Candida albicans-resistant medicines with unsaturated fatty acid structure and preparation method of candida albicans-resistant medicines
A technology of unsaturated fatty acids and Candida albicans, which is applied in the direction of antifungal agents, can solve the problems of high toxicity of bromination reagent liquid bromine, expensive methyl lithium, cumbersome operation, etc., and achieve short synthesis routes, precise routes, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1. In a two-necked round bottom flask (100mL), add sodium azide (0.975g, 15mmol), potassium iodide (0.083g, 0.5mmol), nitrogen protection, add dimethyl sulfoxide 15mL, nitrogen protection, at 50°C Stir. Then, bromooctane (1.92 g, 10 mmol) was added to the reaction system. After the dropwise addition, continue to stir at 50°C for 10h. The reaction was quenched with ice water, extracted with petroleum ether, the organic phase was washed with water and saturated sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated to obtain 1.3 g of octane azide with a yield of 87%.
[0029] Step 2. Add sodium ascorbate (0.17g, 0.85mmol) into a two-neck round bottom flask (100mL) under nitrogen protection. Then 3 mL of ethanol was added. Nitrogen was blown, and 3 mL of an aqueous solution of copper sulfate (0.1 g, 4 mmol) was added, and then propiolic acid (0.73 g, 10 mmol) and octane azide (1.35 g, 8.7 mmol) were added dropwise to react for 5 h. The rea...
example 2- example 6
[0031] Example 2-Example 6, method is the same as Example 1, and the specific experimental consumption is as follows:
[0032] Step 1
[0033] R 1 R 1 Br (g, mmol) NaN 3 (g, mmol) KI (g, mmol) DMSO (mL) R 1 N 3 (g) Yield(%) 2 C 6 h 13 1.64,10 0.975,15 0.083,0.5 15 0.9 70 3 C 6 h 12 Oh 1.80,10 0.975,15 0.083,0.5 15 0.9 63 4 C 12 h 25 2.50,10 0.975,15 0.083,0.5 15 1.4 70 5 C 8 h 17 1.92,10 0.975,15 0.083,0.5 15 1.4 90 6 C 8 h 17 1.92,10 0.975,15 0.083,0.5 15 1.3 84
[0034] Step 2
[0035]
[0036] characterizing data
[0037] Product 2:
[0038]
[0039] 1 H NMR (400MHz, DMSO) δ (ppm): 11.0 (s, 1H), 8.68 (s, 1H), 4.45–4.31 (m, 2H), 1.87–1.73 (m, 2H), 1.22 (pd, J= 8.0,5.0Hz,6H),0.82(t,J=7.0Hz,3H).
[0040] Product 3:
[0041]
[0042] 1 H NMR (400MHz, DMSO) δ (ppm): 11.2 (s, 1H), 8.27 (s, 1H), 4.34 (t, J = 14.1, 7.2Hz, 2H), 1.80 (m, J = 31.2, 23.7Hz ,2H),1.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com