Electrochemical preparation method of graphene
A graphene and electrochemical technology, applied in the field of electrochemical preparation of graphene, can solve the problems of slow chemical preparation process, high quality of graphene without impurities, high preparation cost and difficulty, and achieve low preparation cost and difficulty, and low price Low cost and low power consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
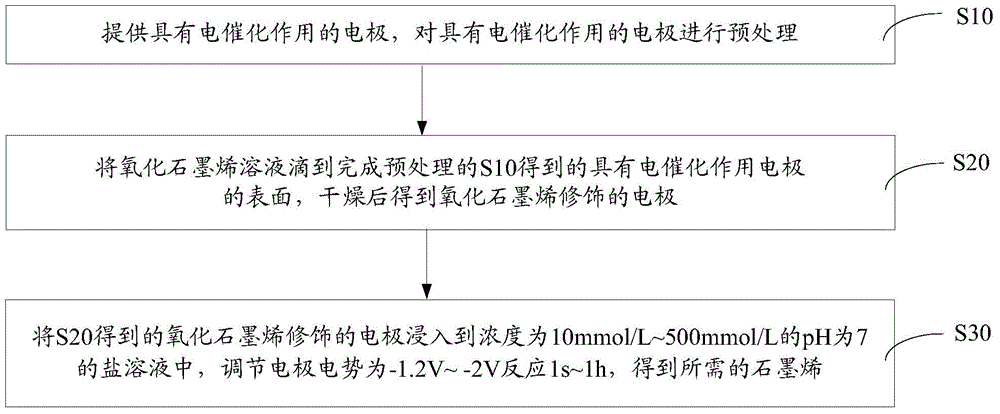
[0024] Such as figure 1 The electrochemical preparation method of the graphene of one embodiment shown, comprises the steps:
[0025] S10, providing an electrode with electrocatalysis, and performing pretreatment on the electrode with electrocatalysis.
[0026] The surface of the electrode with electrocatalysis is provided with a coating with catalytic deoxidation effect. Specifically, the electrode with electrocatalysis can be an electrodeposited copper modified glassy carbon electrode, an electrodeposited nickel modified glassy carbon electrode or an electrodeposited copper nickel modified glassy carbon electrode.
[0027] Alternatively, the material of the electrode with electrocatalysis has a catalytic deoxygenation effect. Specifically, the electrode with electrocatalysis can be a copper electrode, a nickel electrode or a copper-nickel alloy electrode. The operation for pretreatment of the electrode is as follows: the electrode with electrocatalysis is polished and pol...
Embodiment 1
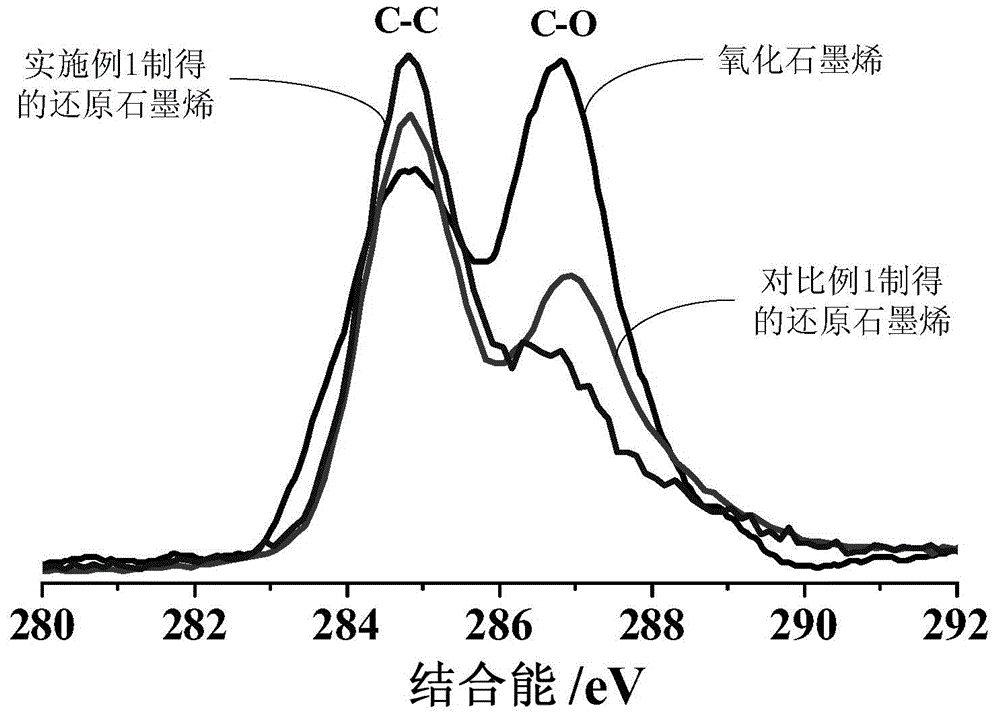
[0039] The graphene oxide prepared by the Modified Hummers method was dispersed in water, and peeled off in a 500W ultrasonic device for 2 hours to obtain a graphene oxide solution with a concentration of 1.2 mg / mL.
[0040] A copper electrode with a diameter of 3 mm was polished and polished with aluminum oxide with a particle size of 50 nm to 0.3 μm, and then ultrasonically cleaned with ethanol and distilled water to complete the pretreatment of the copper electrode.
[0041] 6 μL of a graphene oxide solution with a concentration of 1.2 mg / mL was dropped onto the surface of the pretreated copper electrode, and then the copper electrode was dried at room temperature to obtain a graphene oxide-modified copper electrode.
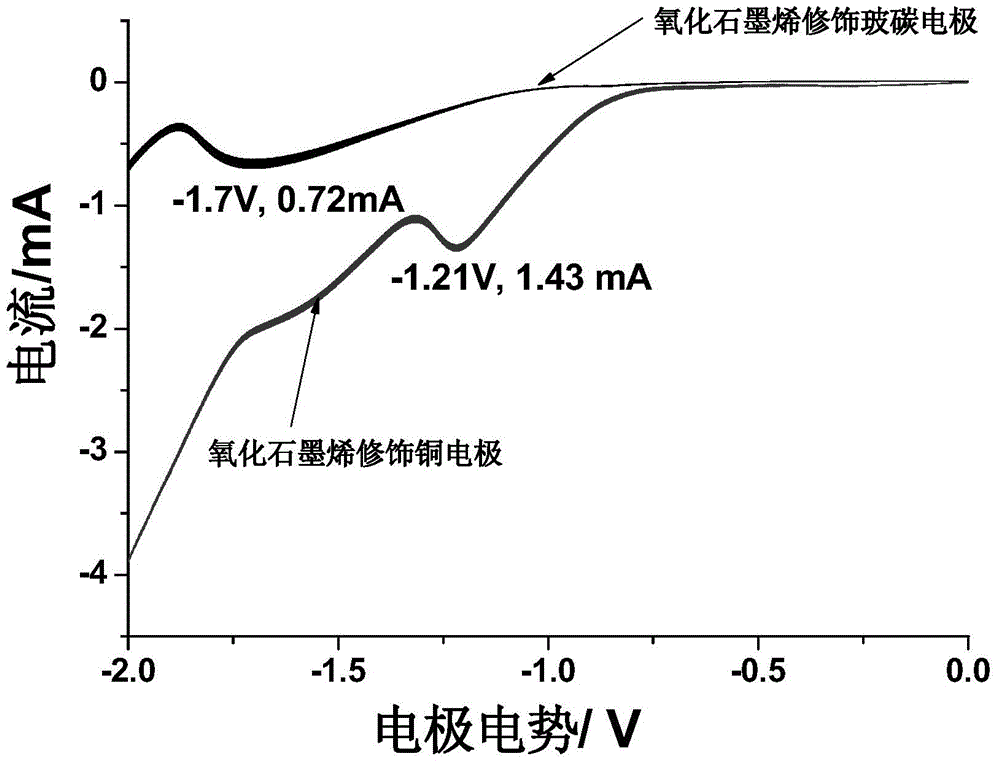
[0042] The graphene oxide-modified copper electrode was immersed in 0.1mol / L Na 2 SO 4 solution, and then perform a linear voltammetric sweep over the potential range of 0 to -1.5V. A cathodic current was evident at a potential of -0.7V and peaked at -1.2V....
Embodiment 2
[0044] The graphene oxide prepared by the Modified Hummers method was dispersed in water, and peeled off in a 500W ultrasonic device for 2 hours to obtain a graphene oxide solution with a concentration of 1.2 mg / mL.
[0045] A glassy carbon electrode with a diameter of 3 mm was polished by alumina with a particle size of 50 nm to 0.3 μm, and then ultrasonically cleaned with ethanol and distilled water.
[0046] Immerse the glassy carbon electrode in 0.5mol / L CuSO 4 In the solution, copper is electrodeposited in the range of 0V to -0.6V to obtain a copper-modified glassy carbon electrode.
[0047] 6 μL of graphene oxide solution with a concentration of 1.2 mg / mL was dropped onto the surface of the copper-modified glassy carbon electrode, and then dried at room temperature to obtain a graphene oxide-copper-modified glassy carbon electrode.
[0048] Immerse the graphene oxide-copper modified glassy carbon electrode in 0.1mol / L Na 2 SO 4In the solution, a linear voltammetry swe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com