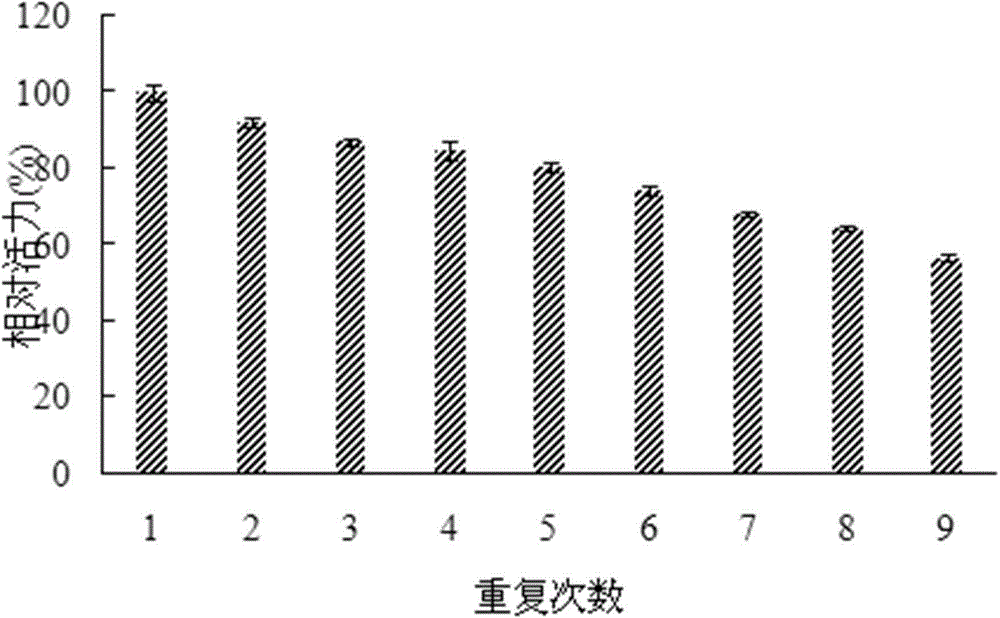
Aryl sulfatase gene, protein encoded by aryl sulfatase gene as well as immobilization method and application of protein
A technology of arylsulfatase and arylsulfate, applied in the field of enzyme engineering, can solve the problems of insufficient enzyme activity, restriction, and low level, and achieve the effects of high enzyme activity, pollution reduction, and water conservation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Amplification and cloning of arylsulfatase gene
[0032] The forward primer sequence of the arylsulfatase gene is: 5'-CGC GGATCC CAAAAAATTAGTATTAT-3’ (underlined as Bam HI recognition sequence), the reverse primer sequence is: 5'-CCC AAGCTT GCGTTTTAGTTCGTAAC-3' (underlined as Hin d III recognition sequence). by Pseudoalteromonas carrageenovora Genomic DNA was used as a template to amplify the arylsulfatase gene.
[0033] Amplification reaction program: pre-denaturation at 95°C for 3 min, denaturation at 94°C for 1 min, annealing at 55°C for 45 s, extension at 72°C for 1 min, 30 cycles; 72°C for 10 min. The size of the PCR product was detected by agarose gel electrophoresis, the purified PCR product was ligated with the pMD18-T vector, and the ligated product was transformed E. coliDH5α competent cells. The cells were smeared on LB culture plates (containing 100 μg / mL ampicillin). After colony PCR identification, the target gene sequence was ...
Embodiment 2
[0034] Embodiment 2: the extraction of arylsulfatase
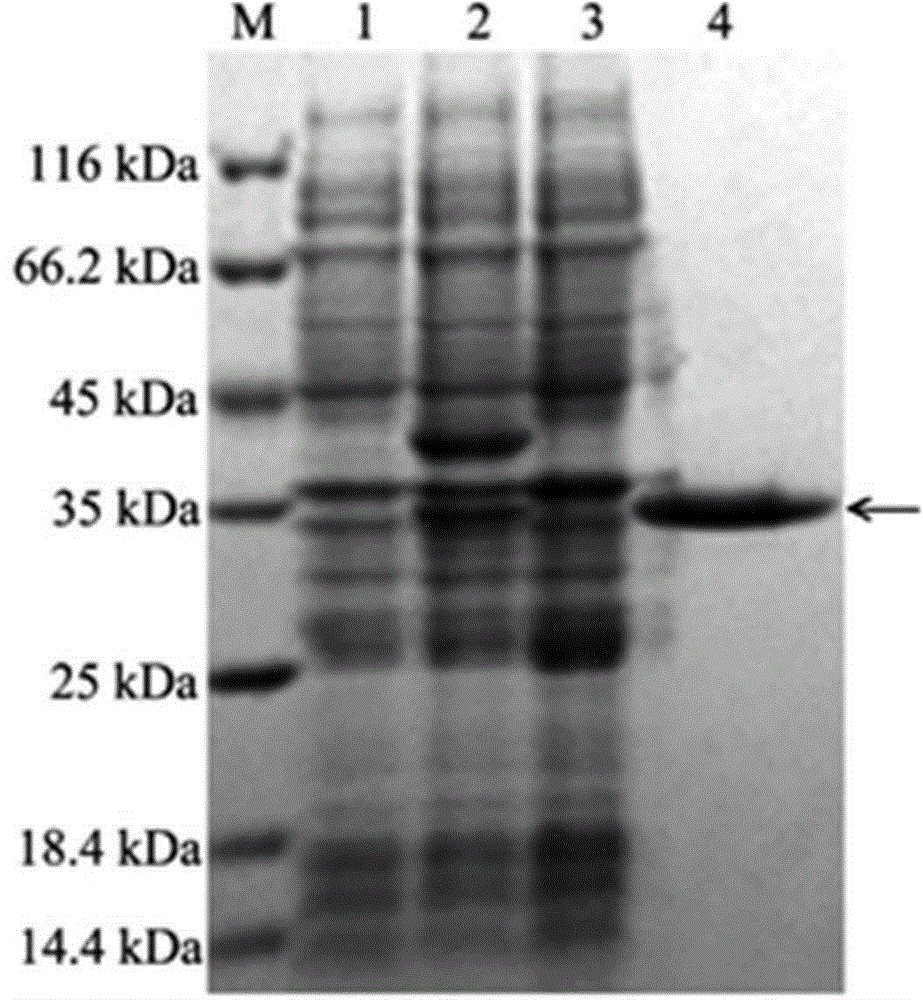
[0035] Transform the recombinant plasmid pET-28a(+) containing the arylsulfatase gene into competent cells E. coli BL21(DE3), cultured on LB plate medium containing 50 μg / mL kanamycin at 37°C for 12 h, selected positive clones and placed them in 5 mL LB liquid medium (containing 50 μg / mL kanamycin ), cultured at 37°C to OD 600 =0.8, add isopropylthio-β-D-galactoside (IPTG) to a final concentration of 50 mmol / L, induce at 15°C for 26 h, collect the cells by centrifugation, and suspend in 200 mL Bacterial cells were lysed by sonication in 50 mM pH 7.0 Tris-HCl buffer. Centrifuge at 15,000×g for 20 min at 4°C to collect the supernatant, which is the crude enzyme solution of arylsulfatase. SDS-PAGE technology was used to analyze the expression and purification of arylsulfatase gene in Escherichia coli, and the results were as follows figure 1 shown.
Embodiment 3
[0036] Example 3: Preparation of Carboxyl Functionalized Magnetic Nanoparticles
[0037] FeCl 3 ·6H 2 O solution and FeCl 2 4H 2 O solution was mixed, ammonia water was quickly added under vigorous stirring conditions, and oleic acid was added dropwise after 1 minute, and rapid stirring was continued for 1 hour at 70°C. After the reaction, a black sol-like substance was obtained, and the resulting precipitate was separated from the reaction system by applying an external magnetic field. Wash with ethanol twice to remove excess oleic acid, and then wash with deionized water to about pH=7. Then add KMnO 4 The solution was ultrasonically oscillated by an ultrasonic cleaner for 8 h, and after magnetic separation, it was washed 3 times with deionized water to obtain a magnetic fluid. Vacuum freeze-drying for 24 h to obtain magnetic nanoparticles with surface-modified carboxyl groups.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com