Method for measuring nano-gold mimetic peroxidase based urease and inhibitor thereof
A technology of peroxidase and determination method, which is applied in the direction of material analysis through observation of the influence on chemical indicators, preparation of test samples, analysis through chemical reaction of materials, etc., which can solve environmental pollution and economic losses and other problems, to achieve the effect of high detection sensitivity, low detection cost and simple detection steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1 milliliter of 0.1 g / L chloroauric acid solution was dissolved in 100 milliliters of water, 3 milliliters of 0.1 g / L trisodium citrate solution was added rapidly after heating and boiling under reflux, the reaction solution changed from light yellow to wine red, and continued to reflux for 15 After 1 minute, the reaction solution was slowly cooled to room temperature. The obtained gold nanoparticles had a diameter of 13 nm and a concentration of 3.1 nmoL / L, and were stored at 4 °C. All glassware used in the above process was soaked in aqua regia, washed thoroughly with double distilled water, and dried.
Embodiment 2
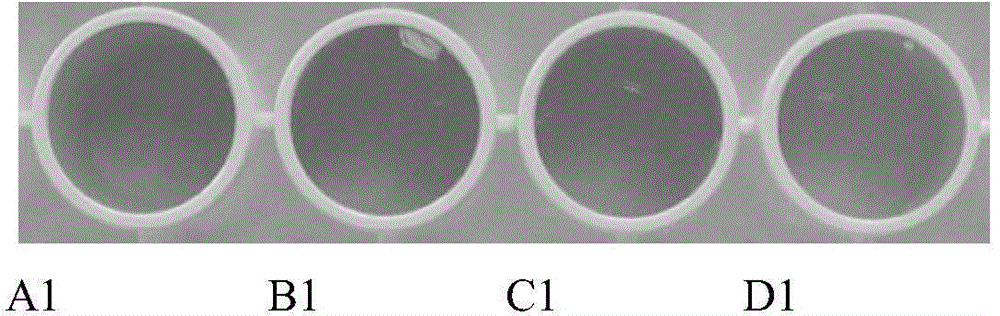
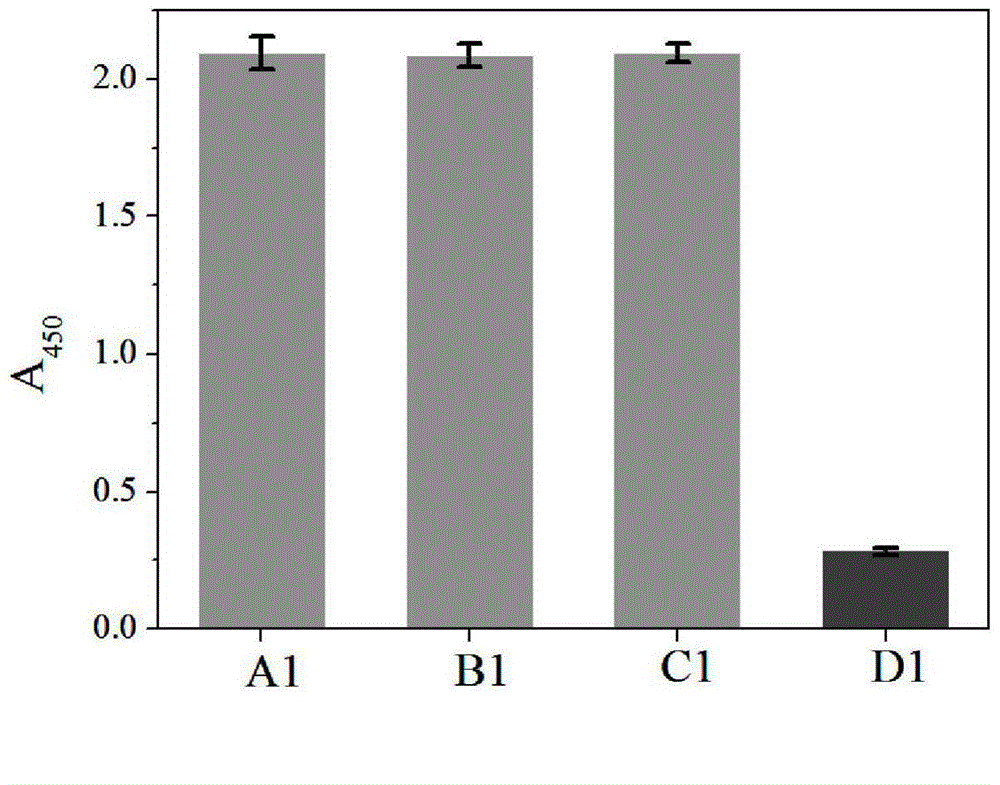
[0036] Add 0.05 mL of 18 U / mL urease solution and 0.1 mL of 5 mmoL / L urea solution into 0.5 mL of phosphate buffer (10 mmol / L, pH=6.40), shake well and incubate at 37°C for 30 minutes. After the reaction, add 0.2 mL of 30% (m / m) hydrogen peroxide solution, 0.05 mL of 3,3',5,5'-tetramethylbenzidine hydrochloride solution with a concentration of 16 mmol / L and 0.10 mL of the nano-gold solution prepared in Example 1 was mixed evenly and then incubated at 37° C. for 10 minutes. Immediately add 0.2 mL of 20% (V / V) sulfuric acid solution to terminate the reaction, visually observe the color change or measure the absorbance value A 450 . When visually observing the color change, the color of the solution in the control group was all dark yellow, and the color of the color development system in the experimental group became light yellow (see figure 1 ). Assay A 450 , the control group A 450 Almost no change, experimental group A 450 significantly reduced (see figure 2 )
Embodiment 3
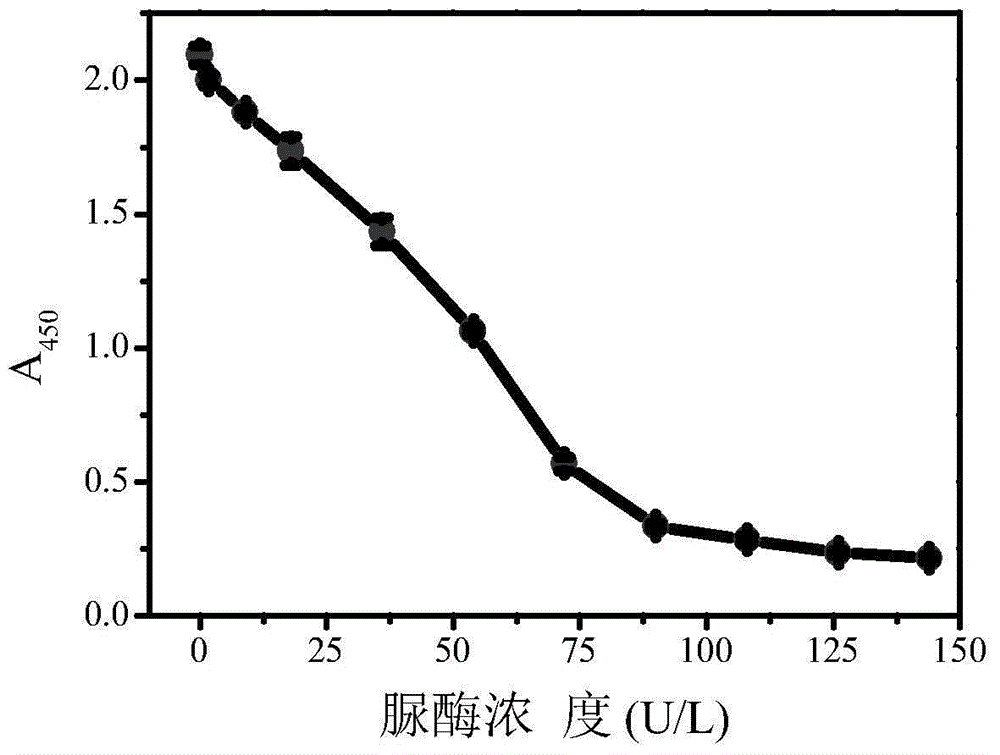
[0038] Add 0.05 mL of urease solution of different concentrations and 0.1 mL of 0.5 moL / L urea solution into 0.5 mL of phosphate buffer (10 mmol / L, pH=6.40), shake well and incubate at 37°C for 30 minutes. After the reaction, add 0.2 mL of 30% (m / m) hydrogen peroxide solution, 0.05 mL of 3,3',5,5'-tetramethylbenzidine hydrochloride solution with a concentration of 16 mmol / L and 0.10 mL of the nano-gold solution prepared in Example 1 was mixed evenly and then incubated at 37° C. for 10 minutes. Immediately add 0.2 mL of 20% (V / V) sulfuric acid solution to terminate the reaction, and measure the absorbance value A 450 . Depend on image 3 It can be seen that with the increase of urease concentration, A 450 value decreases gradually. In the range of 1.8 ~ 90 U / L, A 450 The value has a linear relationship with the concentration of urease, and the detection limit is 1.8 U / L (see Figure 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com