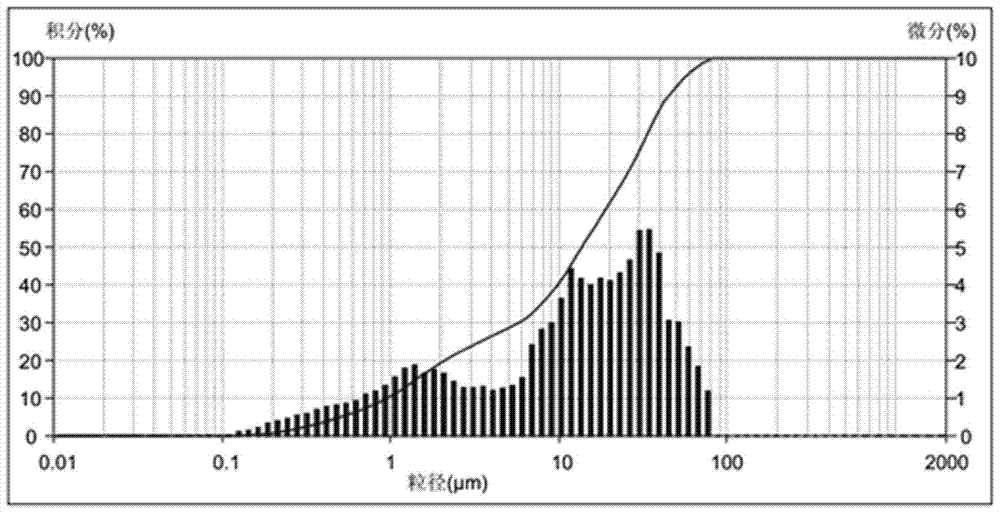
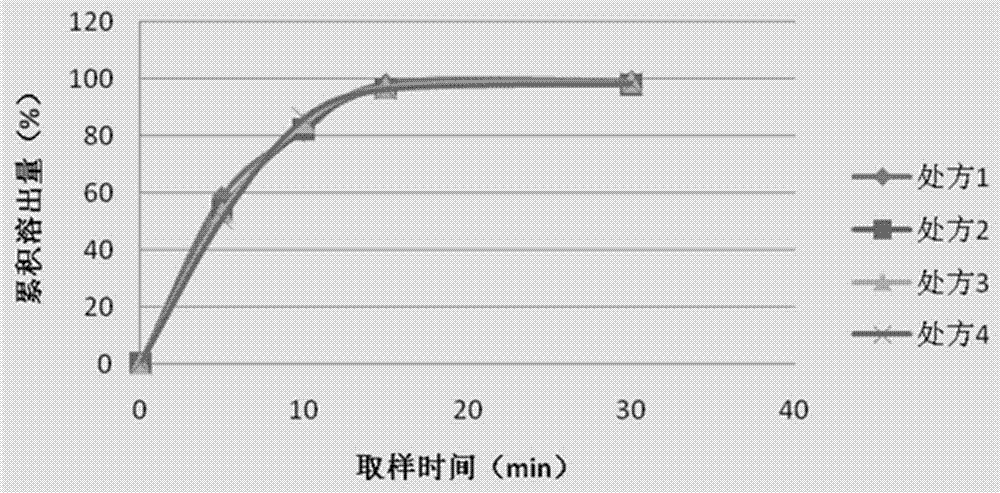
A high-loading, fast-dissolving ferric citrate composition and its preparation method
A technology of ferric citrate and high drug loading, applied in drug combination, drug delivery, pharmaceutical formulation, etc., to achieve the effect of product quality satisfaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] formula1234 Iron citrate hydrate 1200mg 1200mg 1200mg 1200mg Powdered cellulose 100mg 110mg 120mg 130mg corn starch 30mg 10mg 20mg 10mg Gelatin-xanthan gum mixture 5mg 5mg 5mg 5mg Pure water Right amount Right amount Right amount Right amount Calcium stearate 30mg 30mg 30mg 30mg Premixed coating powder Right amount Right amount Right amount Right amount
[0036] .
[0037] Weigh the materials according to formula 1, crush them mechanically, pass through a 100-mesh sieve, and prepare them for use; prepare the gelatin-xanthan gum mixture with purified water; combine the mechanically crushed iron citrate hydrate and powdered cellulose in multiple Mix in a sports mixer for 20 minutes, collect the materials, and set aside.
[0038] Put the mixed materials in a high-efficiency wet granulator, shear at high speed for 60 seconds, stop high-speed shear, turn on low-speed shear, add gelatin-xanthan gum mixture aqueous solution for granulation, and adjust the binder according...
Embodiment 2-4
[0043] Weigh the materials according to formula 2-4, and the preparation method is the same as in Example 1.
Embodiment 5
[0044] Example 5 In vitro dissolution measurement method
[0045] Dissolution method: slurry method, 100 revolutions / min; solvent: pH 1.2 solution (sodium chloride 2.0g, concentrated hydrochloric acid 7ml, add water to 1000ml) 1000ml; time: 15min, Q value ≥90%.
[0046] Concentration of reference solution: 0.45mg / ml.
[0047] Drug concentration detection method: UV method; 220nm.
[0048] Evaluation method of other indicators: ChP2010. Two Appendix IB.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com