Gradient micro-droplet array forming method based on sequential injection and microfluidic technology
A microfluidic technology and sequential injection technology, applied in the field of gradient micro-droplet array formation, can solve the problems of sample waste, limited application, low reproducibility of screening system, etc., and achieve simple device and principle and high degree of automation , the effect of saving experimental cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
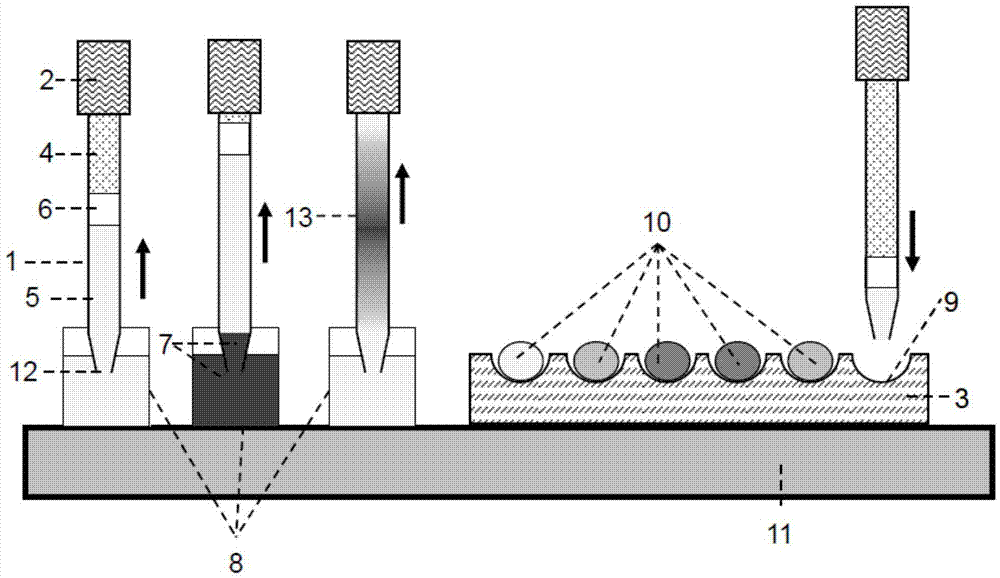
[0050] figure 1 It is a schematic diagram of the formation method of gradient micro-droplet array based on sequential injection and microfluidic droplet technology. The specific operation process is as follows: use the capillary 1 as a sampling probe, and connect its tail to the liquid driving system 2 . The sampling port 12 of the capillary 1 is sharpened to reduce cross-contamination during sampling. The inner wall of the capillary 1 and the outer wall of the sampling port 12 are treated with fluorosilanization to prevent the adsorption of samples / reagents on their surfaces. The liquid with low thermal expansion coefficient is filled in the capillary 1 as the carrier stream 4, and the air bubbles in the capillary 1 and the liquid driving system 2 are completely removed. An oil phase 6 immiscible with the sample / diluent is introduced into the sampling port 12 of the capillary 1 to separate the carrier stream 4 and the diluent 5 . The storage tube of the sample / reagent 7 an...
Embodiment 2
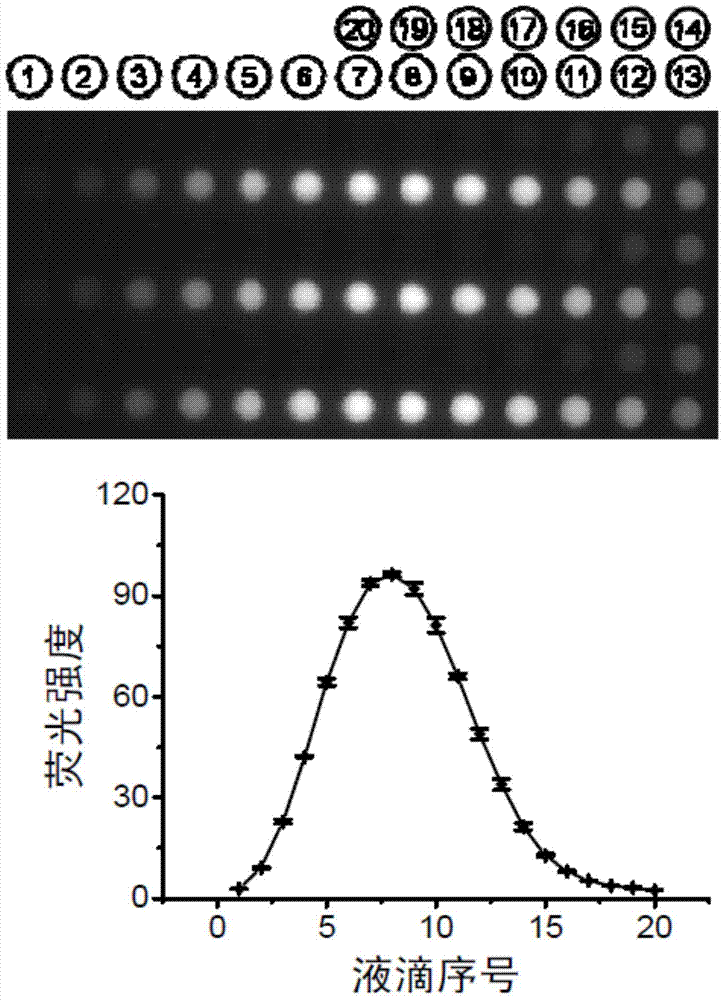
[0052] figure 2 is based on figure 1In the sequential injection gradient method, the micro-droplet array fluorescence pictures and corresponding curves generated by using sodium fluorescein as a model sample. A syringe pump is used as the liquid driving device 2, and a layer of mineral oil is covered on the microwell array chip 3 to avoid the evaporation of micro samples in the microwells. Start the syringe pump, first draw 120 nanoliters of 50 mmol per liter borax buffer as a diluent in capillary 1, then draw 20 nanoliters of 1 mmol per liter sodium fluorescein sample, and then draw 60 nanoliters of Borax buffer. During the extraction process, sodium fluorescein diffuses in the borax buffer to form a sample zone. Then, the sodium fluorescein sample zone in the capillary 1 was spotted onto the microwell array chip 3 to form a droplet array 10 , each droplet had a volume of 9 nanoliters, and a total of 20 droplets were formed. Repeat the above steps 3 times to generate a t...
Embodiment 3
[0054] image 3 is to use figure 1 Schematic diagram of the operation process of quantitative high-throughput protein crystallization condition screening by the sequential injection gradient micro-droplet array method. Firstly, a layer of mineral oil is covered on the microwell array chip 3 to avoid the evaporation of trace samples in the microwells. A certain volume of precipitant solution 14 is extracted in the storage tube 8 of the precipitant solution, and then pushed out into the microwells 9, and the volume of the precipitant droplet in each well is 2 nanoliters. according to figure 1 In the described method, 50 nanoliters of protein buffer solution, 20 nanoliters of protein solution 15 and 50 nanoliters of protein buffer solution are sequentially extracted in the capillary 1, and the protein solution 15 diffuses in the capillary to form a protein zone with a concentration gradient. Inject 2 nanoliters of the protein zone solution into the precipitant solution in each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com