Lmfp cathode materials with improved electrochemical performance
A cathode material and electroactive material technology, applied in the field of olivine lithium manganese iron phosphate cathode material, can solve the problems of low energy and power density, failure to reach the theoretical level, and decrease in specific capacity, and achieve excellent cycle life and enhanced cycle The effect of life, high charging rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Examples 1) to 4) exhibit some specific advantages, especially when prepared by wet grinding. The cathode materials of examples 1) to 4) tend to be less hygroscopic and thus absorb less water. Other advantages of these embodiments may include higher ionic conductivity (possibly due to e.g. Li 3 PO 4 and / or Li 4 P 2 o 7 Lithium salt formation), high charge acceptance, high capacity, and excellent cycle stability.
[0038] In any of the preceding embodiments, the dopant metal is selected from one or more of the following: magnesium, calcium, strontium, cobalt, titanium, zirconium, molybdenum, vanadium, niobium, nickel, scandium, chromium, copper, zinc, beryllium , lanthanum, and aluminum. The dopant metal is preferably magnesium, cobalt, titanium, vanadium, nickel, or aluminum or a mixture of two or more thereof. The dopant metal is more preferably magnesium or a mixture of magnesium and one or more of: calcium, strontium, cobalt, titanium, zirconium, molybdenum, v...
example 1-3
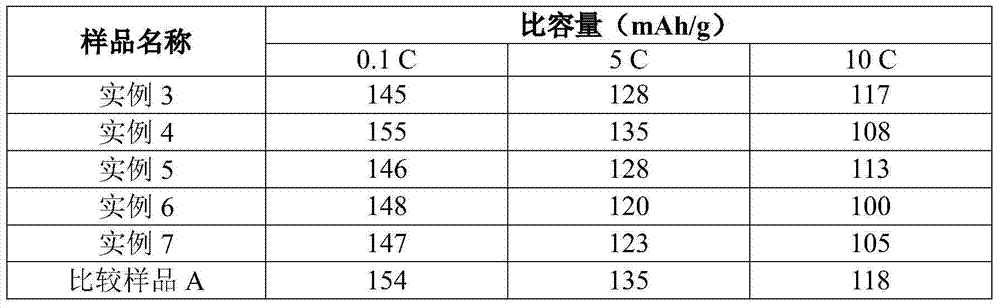
[0064] Examples 1-3 and Comparative Samples A-D
[0065] Examples 1-3 and Comparative Samples A-D were prepared using the solid state method as described in WO 2009 / 144600.
[0066] Table 1
[0067] name
Mode
a+2b+2c+dV
Comparative Sample A
LiMn 0.8 Fe 0.2 PO 4
3.0
Comparative sample B
Li 1.025 mn 0.8 Fe 0.2 PO 4
3.025
Comparative Sample C
Li 1.1 mn 0.71 Fe 0.24 PO 4
3.0
Comparative sample D
Li 1.1 mn 0.76 Fe 0.19 PO 4
3.0
[0068] Example 1
Li 1.1 mn 0.8 Fe 0.1 Mg 0.05 PO 4
3.0
Example 2
Li 1.1 mn 0.8 Fe 0.08 Mg 0.07 PO 4
3.0
[0069] The resulting particles were mixed with vapor-grown carbon fibers and a binder in a weight ratio of 93:2:5 to form an electrode. The electrodes were given the same designations as the respective electroactive materials they contained (as indicated in Table 1 above).
[00...
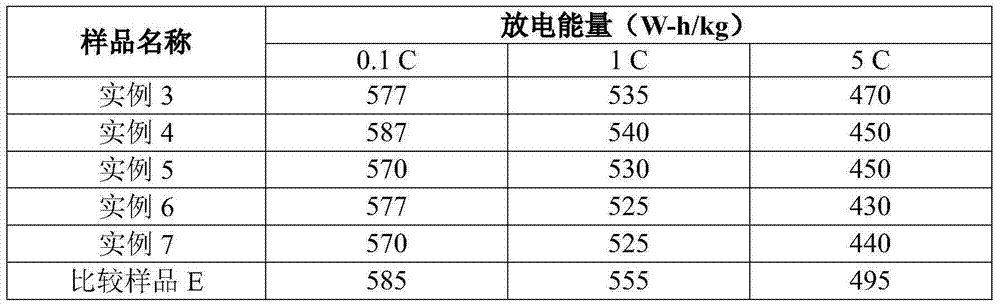
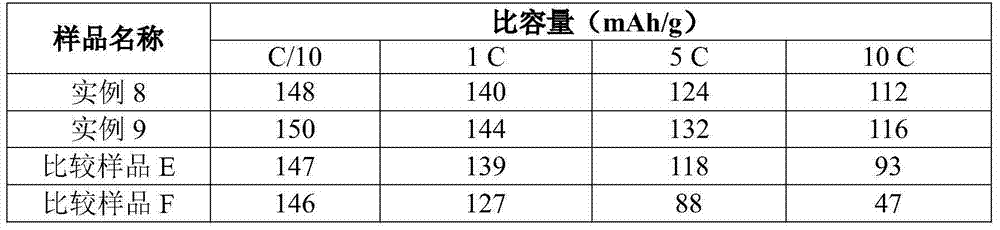
example 3-7
[0074] Examples 3-7 and Comparative Sample E
[0075] Olivine LMFP particles having the formula shown in Table 2 below were prepared using the following method. Slurry ferric oxalate dihydrate (solids) and manganese carbonate (solids) with water in a mixing tank with a high shear mixer (or rotor stator mixer) to a concentration of 35-45% by weight solids . Where a dopant metal is included, the dopant metal precursor is magnesium acetate and / or cobalt acetate. The 85% phosphoric acid was slowly metered into the mixing tank by a pump. Carbon dioxide is released when phosphoric acid reacts with manganese carbonate. After the acid addition was complete, the slurry was mixed for approximately 30 minutes to allow carbon dioxide to continue to off-gas. Then, lithium hydroxide monohydrate (solid) was added to the mixing tank. The slurry went through a viscous phase as the lithium hydroxide was mixed with the solids. LiOH addition was exothermic and the temperature rose to 55-60 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com