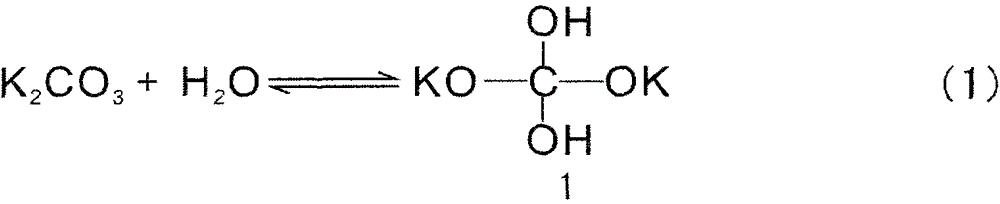
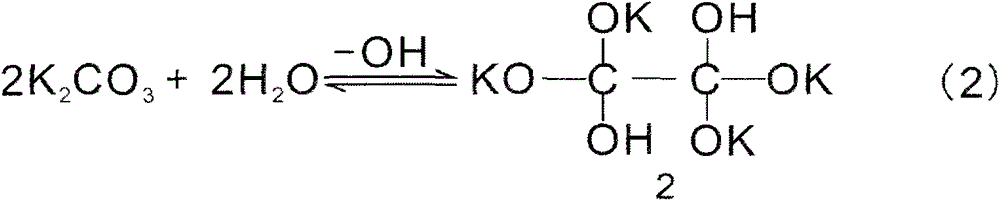
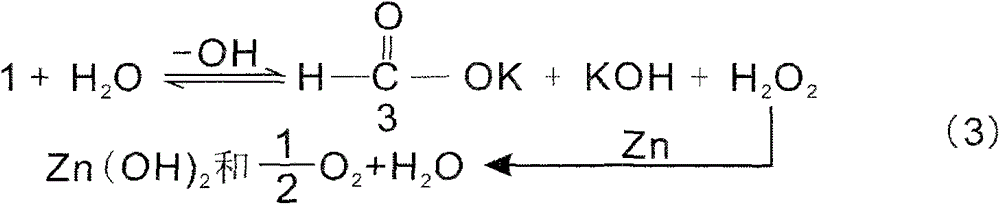Method for reducing inorganic compound by water as hydrogen source or by molten alkali and use thereof
A technology for hydrogen sources and uses, applied in the preparation of organic compounds, the preparation of amino compounds, chemical instruments and methods, etc., can solve problems such as air pollution, governance methods without market economic rules, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Put 100 grams of 30-48% potassium hydroxide or sodium hydroxide or lithium hydroxide or calcium hydroxide aqueous solution or water suspension; or drop 100 grams of 25% tetraalkylammonium hydroxide aqueous solution in the reactor, and put 10-20 grams of zinc powder or Raney nickel or reduced iron powder, start stirring, control the temperature below 40°C, slowly add CO 2Or carbonate or formate or formaldehyde 5-10 grams each, after stirring and reacting for 1-2 hours, gradually raise the temperature to 90°C, control the temperature at 90-220°C, continue stirring and reacting for 14-24 hours to generate glycolic acid and acetic acid Mixed alcohol of diol+ethanol+methanol, CO 2 Or the conversion rate of carbonate or formate or formaldehyde is more than 95%, and the total yield of glycolic acid and mixed alcohol is more than 90%.
[0065] Then filter out the metal solids under anaerobic conditions and store them in a concentrated alkali solution for the next batch of use....
Embodiment 2
[0067] Put 100 grams of 25% tetraethylammonium hydroxide aqueous solution and 5 to 20 grams of magnesium powder or Raney nickel into the reactor, start stirring, control the temperature at -10 to 140°C, and slowly add 5 to 10 grams of CO 2 Or potassium carbonate or potassium formate, stirred for 4 to 48 hours, then slowly added with CO 2 Or potassium carbonate or potassium formate equimolar calcium hydroxide, down to room temperature, filter out solid matter, separate out metallic magnesium solid matter simultaneously, generate calcium glycolate solid, conversion rate is more than 95%, the yield of calcium glycolate is More than 85%; then use sulfuric acid to neutralize calcium glycolate, and concentrate to obtain 50% glycolic acid aqueous solution.
Embodiment 3
[0069] Put 100 grams of 30-48% potassium hydroxide or sodium hydroxide or lithium hydroxide aqueous solution, or put 100 grams of 25% tetramethylammonium hydroxide aqueous solution, and add 10-20 grams of zinc powder or Raney nickel at the same time, start Stir, control the temperature at 10-80°C, control the pressure at 1-4MPa, slowly add 20-40 grams of ammonium bicarbonate; or slowly add 10-20 grams of CO 2 and 20-40 grams of ammonia gas, heat preservation reaction for 4-48 hours to generate glycine, the conversion rate is 99%, and the yield of glycine is more than 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com