Preparation method for treprostinil intermediate
A technology of molar weight and compound, applied in the direction of organic chemistry, bulk chemical production, etc., can solve problems such as environmental pollution, instability of aldehyde-based compounds, harsh reaction conditions, etc., and achieve broad industrial application prospects, easy recrystallization, purification, and reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
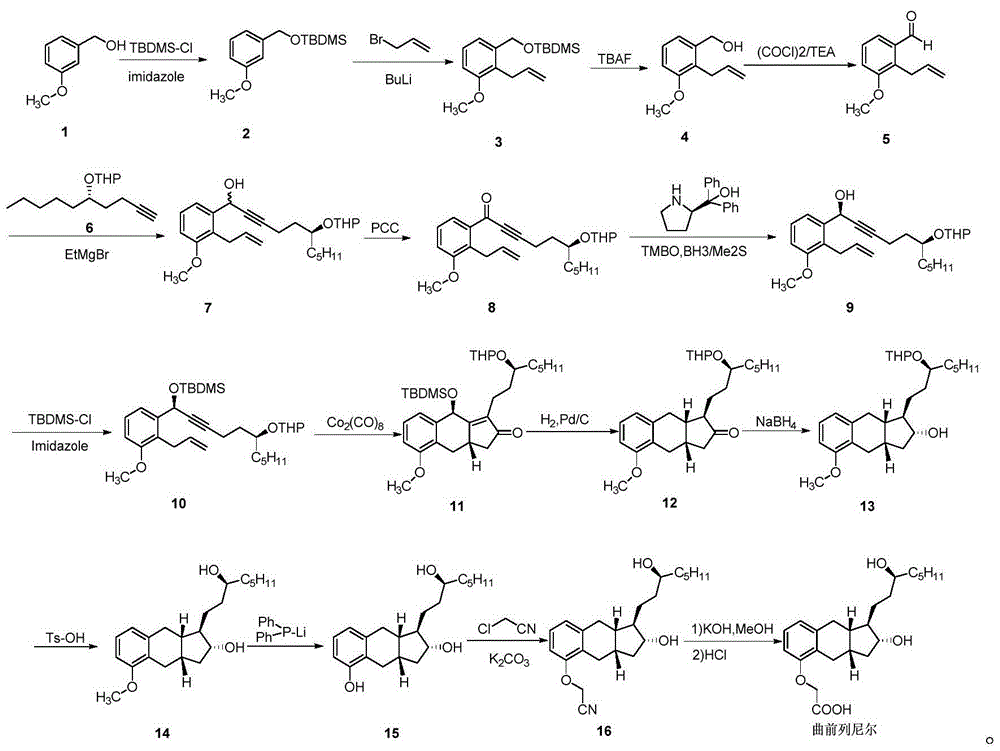
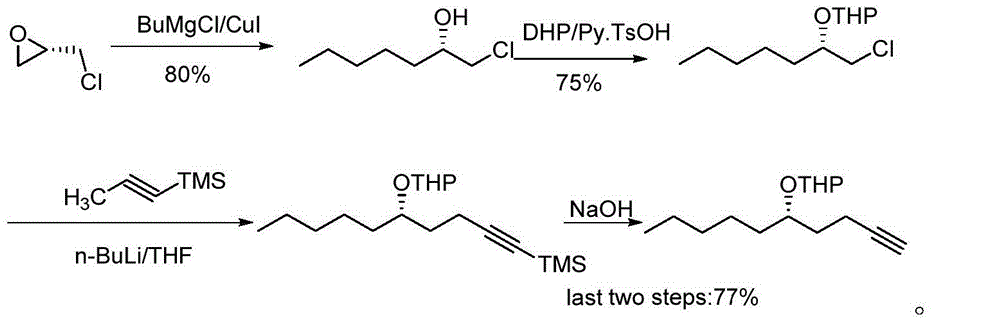
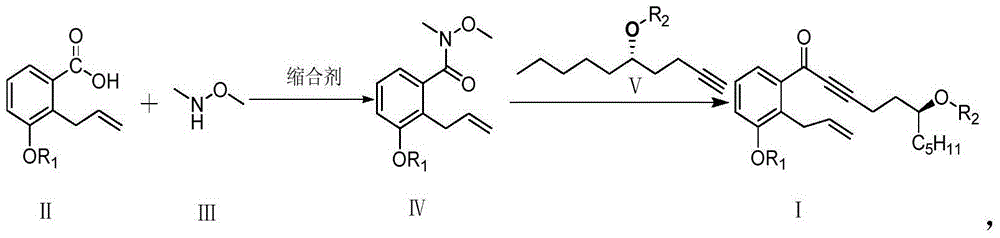
Image
Examples
Embodiment 1
[0110]
[0111] Under the protection of nitrogen, add 1000ml of methanol to the reaction flask, and then add the compound 3-hydroxy-benzoic acid methyl ester 200.0g (1.314mol), potassium carbonate 218.0g (1.580mol), sodium iodide 20.0g (0.133mol) 191.0 g (1.579 mol) of bromopropylene, heated to reflux; after stirring for 7-9 hours, TLC detected that the reaction of raw materials was complete (developing agent: petroleum ether: ethyl acetate = 4:1), cooled to 25 ° C, and reacted 500ml of methyl tert-butyl ether was added to the residue, filtered, the filtrate was washed once with 300ml of 15% aqueous ammonium chloride solution, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain Light yellow oil 3-allyloxy-benzoic acid methyl ester 243.5g (1.267mol, molar yield: 96.4%, HPLC purity 96.5%).
Embodiment 2
[0113]
[0114] Put 243.5g (1.267mol) of 3-allyloxy-benzoic acid methyl ester in the reaction flask, raise the temperature to 220°C for 1.0-3.0 hours, then cool down to 60°C; add 500ml of methyl tert-butyl ether and 844ml 1mol / L lithium hydroxide aqueous solution, adjust the temperature to 30-40°C and stir the reaction overnight, cool down to 0-5°C, add 117ml concentrated hydrochloric acid dropwise to adjust the pH of the reaction solution to 1-2, and use 700ml formazan extracted with tert-butyl ether, the organic phase was washed successively with saturated aqueous sodium bicarbonate solution (700ml×2), saturated aqueous sodium chloride solution (500ml), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to dryness to obtain light yellow half Solid 2-allyl-3-hydroxy-benzoic acid methyl ester 193.8g (1.008mol, molar yield 79.6%, HPLC purity 96.4%).
Embodiment 3
[0116]
[0117] 193.8g (1.008mol) of 2-allyl-3-hydroxyl-benzoic acid methyl ester was placed in the reaction flask, 3000ml of acetone was added, and then 14.8g (0.099mol) of sodium iodide and 393.4g of cesium carbonate ( 1.207mol), benzyl bromide 190.6g (1.112mol), and the temperature was raised to reflux. Stir the reaction for 1-2 hours, TLC analysis of the raw material reaction is complete (developer: petroleum ether: ethyl acetate = 4:1), cool down to room temperature, filter, concentrate to dryness under reduced pressure; add 300mL of n-hexane, water (200ml*3 ) washed, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to dryness to give light yellow oily matter 2-allyl-3-benzyloxy-methyl benzoate 279.8g (0.991mol, molar yield 98.3% , HPLC purity 91.1%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com