Metal catalyst and its preparation method and its application in the selective hydrogenation of benzene to cyclohexene
A metal catalyst and catalyst technology, applied in the direction of hydrogenation to hydrocarbons, etc., can solve the problems of difficult separation of catalyst and product, expensive ruthenium catalyst, and reduced cyclohexene selectivity, etc., to achieve inhibition of coalescence, simple preparation process, and improved selectivity. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 0.5430g and 0.0395g of ruthenium acetylacetonate and zinc acetylacetonate respectively, add them into 80ml of diphenyl ether containing 40ml of oleamide, stir and dissolve, heat to 100°C, keep the temperature for 20min, and quickly inject 5.4mL of triethyl ether into the solution A THF solution of lithium borohydride (the concentration of lithium triethylborohydride is 1mol / L), the temperature was raised to 160°C under rapid stirring, and then slowly raised to 180°C, and after reflux for 30min, the reaction flask was removed from the heater. stop responding. After adding an appropriate amount of ethanol and centrifuging, the alloy catalyst with Ru-Zn was obtained, and the obtained catalyst was dispersed in hexane (all experiments were carried out under argon atmosphere).
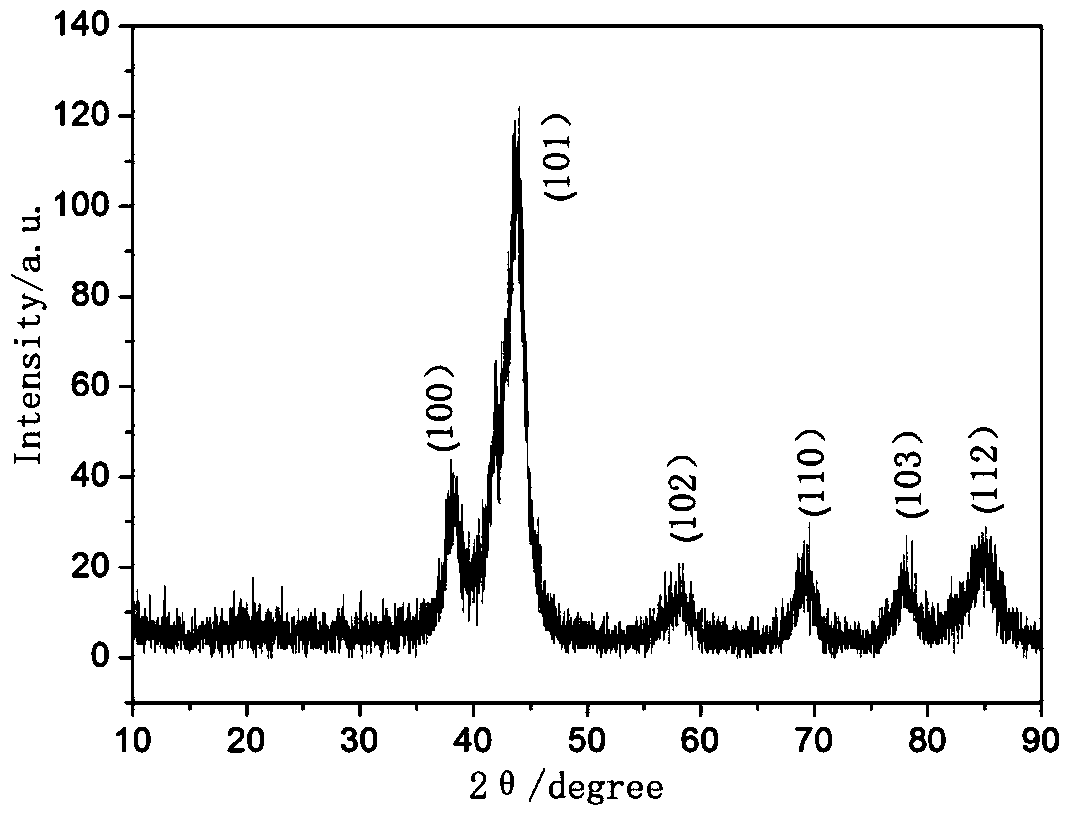
[0030] figure 1 For the XRD picture of the sample prepared for this implementation, the diffraction peaks at the positions of about 38°, 44°, 58°, 69°, and 78° can be determined to be the 100, ...
Embodiment 2
[0032] Weigh 0.607g of ruthenium acetylacetonate and 0.0132g of zinc acetylacetonate respectively, add them into 100ml of diphenyl ether containing 50ml of oleamide, stir and dissolve, heat to 120°C, keep the temperature for 20min, and quickly inject triethyl hydroboration into the solution For lithium, the temperature was raised to 160°C under rapid stirring, and then slowly raised to 180°C. After reflux for 30 minutes, the reaction flask was removed from the heater to stop the reaction. After adding an appropriate amount of ethanol and centrifuging, the alloy catalyst with Ru-Zn was obtained, and the obtained catalyst was dispersed in hexane (all experiments were carried out under argon atmosphere).
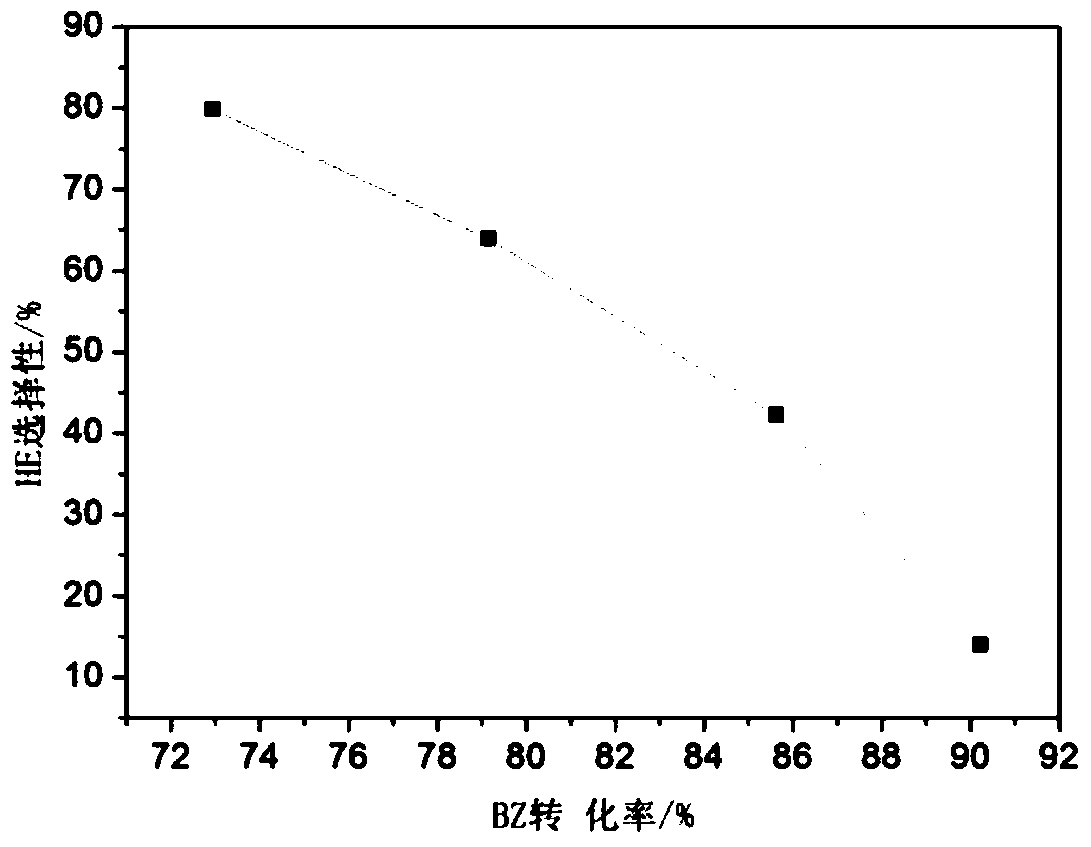
[0033] The catalytic performance of the catalyst to catalyze the hydrogenation of benzene to cyclohexene at a reaction temperature of 140°C is shown in image 3 with Figure 4 ; Catalyst catalytic hydrogenation of benzene to cyclohexene life test when the reaction temperature ...
Embodiment 3
[0035] Weigh 0.575g of ruthenium acetylacetonate and 0.0265g of zinc acetylacetonate respectively, add them into 90ml of diphenyl ether containing 35ml of oleamide, stir and dissolve, heat to 140°C, keep the temperature for 20min, and quickly inject triethyl borohydrogenate into the solution For lithium, the temperature was raised to 180°C under rapid stirring, and then slowly raised to 220°C. After reflux for 30 minutes, the reaction flask was removed from the heater to stop the reaction. After adding an appropriate amount of ethanol and centrifuging, the alloy catalyst with Ru-Zn was obtained, and the obtained catalyst was dispersed in hexane (all experiments were carried out under argon atmosphere).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com