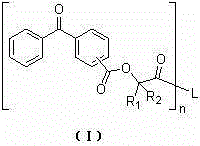
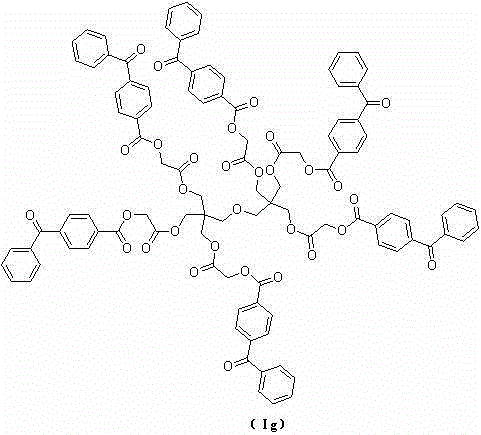
Benzophenone macromolecular photoinitiator and preparation method thereof
A technology of benzophenones and photoinitiators, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of intractable waste water, low migration and high cost, and achieve mild reaction conditions. , Simple operation, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
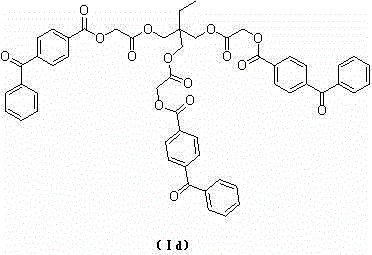
[0042] Example 1: Preparation of 2-benzoyl-benzoyloxy-acetic acid diethylene glycol diester (Ia)
[0043]In a 250ml four-necked flask equipped with mechanical stirring, add 25.0g of diethylene glycol, 100ml of dichloromethane, and 59.6g of triethylamine, cool down to 0~5°C in an ice-water bath, add dropwise 55.9g of chloroacetyl chloride, and control dropwise Acceleration, keep the temperature of the reaction solution at 0~5°C, after the dropwise addition, raise the temperature to 20~25°C and continue stirring for 3 hours, and control the monoester content in GC to 1 H NMR (CDCl 3 ) δ: 8.82 (m, 1H), 8.56 (m, 1H), 8.43 (m, 1H), 7.90 (d, 1H), 7.75 (m, 2H), 7.61 (m, 1H), 5.37 (m, 1H ), 3.76 (s, 3H), 1.62 (d, 3H).
Embodiment 2
[0044] Example 2: Preparation of 2-benzoyl-benzoyloxy-acetic acid diethylene glycol diester (Ia)
[0045] In a 250ml four-necked bottle equipped with mechanical stirring, add 25.0g of diethylene glycol, 100ml of dichloromethane, 59.6g of triethylamine, cool down to 0~5°C in an ice bath, add dropwise 55.9g of chloroacetyl chloride, and control dropwise Acceleration, keep the temperature of the reaction solution at 0~5°C, after the dropwise addition, raise the temperature to 20~25°C and continue stirring for 3 hours, and control the monoester content in GC to <1.0%. Add 40ml of water to the reaction solution, separate after stirring, wash the organic phase twice with 40ml of saturated sodium bicarbonate, once with 40ml of saturated brine, once with 40ml of water, and transfer to another machine equipped with mechanical stirring after removing the solvent. In a 1000ml four-neck flask, add 400ml N,N'-dimethylformamide, 106.5g 2-benzoylbenzoic acid, 55.0g sodium carbonate, heat to ...
Embodiment 3
[0046] Example 3: Preparation of 2-benzoyl-benzoyloxy-butylene glycol diester (Ib)
[0047] In a 250ml four-neck flask equipped with mechanical stirring, add 20.0g 1,4-butanediol, 100ml tetrahydrofuran, 71.7g diisopropylethylamine, cool down to 0~5℃ in an ice bath, and dropwise add 52.6g Chloroacetyl chloride, control the rate of addition, keep the temperature of the reaction solution at 0~5°C, raise the temperature to 20~25°C after the dropwise addition and continue stirring for 4 hours, and control the monoester content in GC to 1 H NMR (CDCl 3 ) δ: 8.82 (m, 1H), 8.56 (m, 1H), 8.43 (m, 1H), 7.90 (d, 1H), 7.75 (m, 2H), 7.61 (m, 1H), 5.37 (m, 1H ), 3.76 (s, 3H), 1.62 (d, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pencil hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com