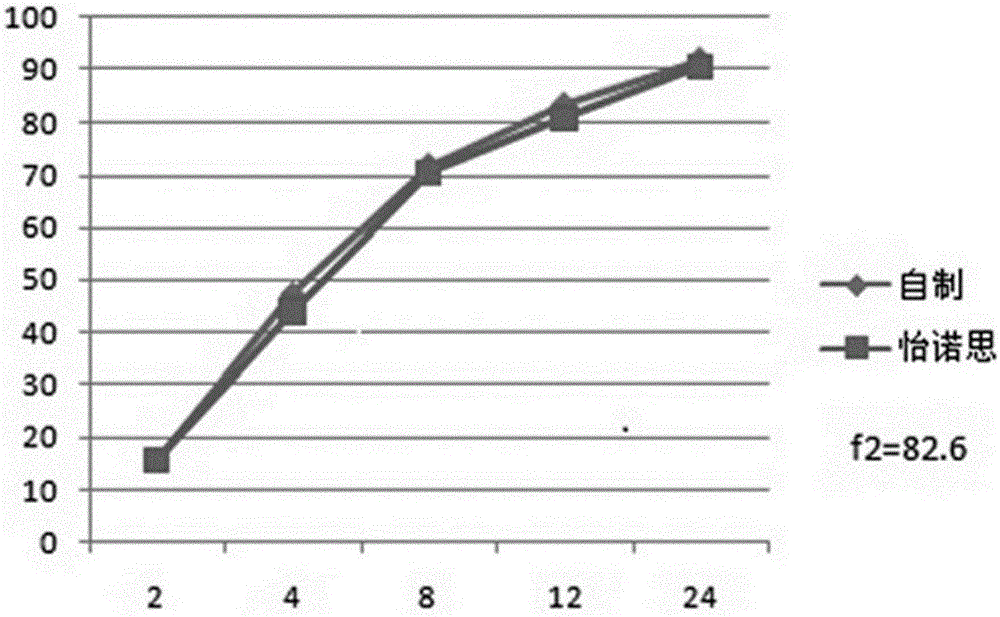
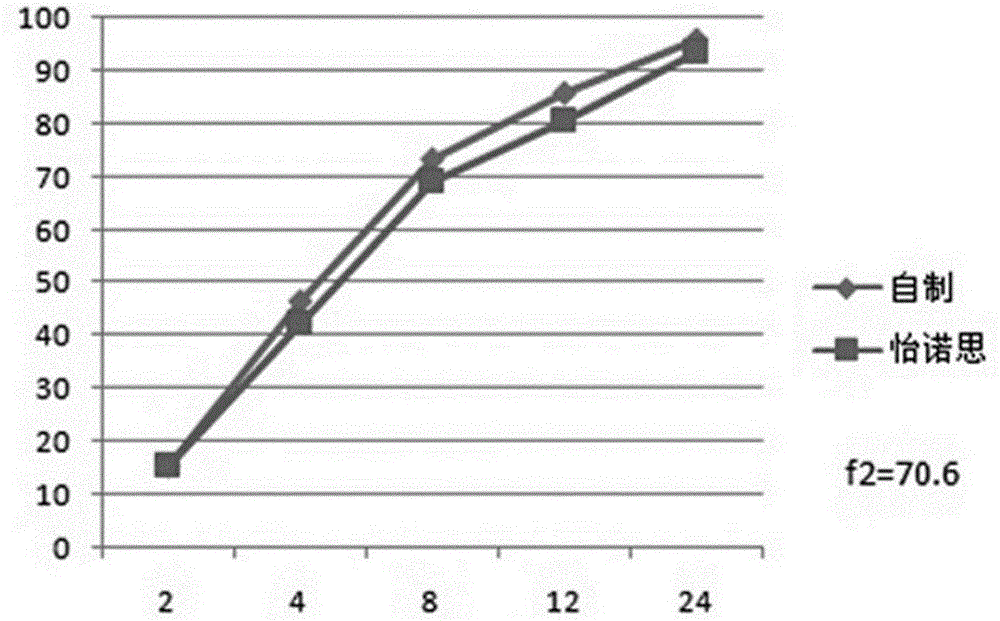
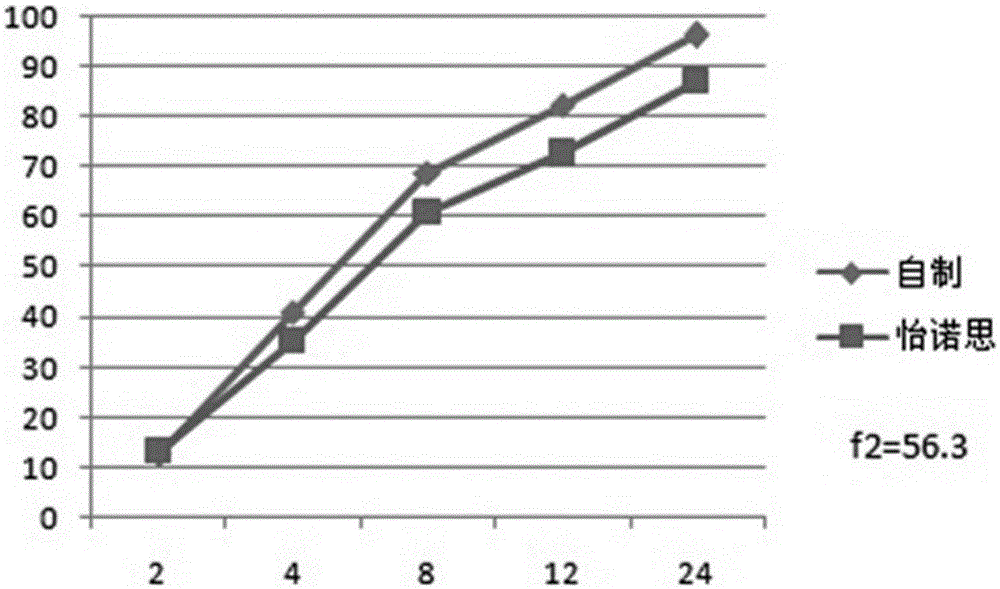
Venlafaxine hydrochloride long-acting controlled-release pellets and preparation method thereof
A technology of venlafaxine hydrochloride and sustained-release pellets, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve the problems of low bioavailability, complicated preparation process, and Conformity and other issues, to achieve the effect of clear drug effect, mature preparation process, and ensure uniform distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 venlafaxine hydrochloride sustained-release pellets
[0055] 1. Prescription of pills: 2000 pills (1000 pills for the first pill and 1000 pills for the second pill)
[0056] material
Prescription volume (g)
Proportion (%)
effect
200
/
Ball core
180
About 90% of the ball core
14.4
About 8% of active ingredients
Antisticking agent
5.4
About 3% of active ingredients
85% ethanol
Appropriate amount
/
[0057] In the above prescription, the particle size of the pellet core is 500-700 μm, of which the pellet core with a particle diameter of 500-600 μm accounts for 50%, and the talcum powder is 3000 mesh.
[0058] 2. Disposal of the first sustained-release pills: based on 1000 pills
[0059] material
...
Embodiment 2
[0070] The preparation of embodiment 2 venlafaxine hydrochloride sustained-release pellets
[0071] 1. Prescription of pills: 2000 pills (1000 pills for the first pill and 1000 pills for the second pill)
[0072] material
Prescription volume (g)
Proportion (%)
effect
200
/
Ball core
160
About 80% of the ball core
Talc powder
16
About 10% of the active ingredient
Antisticking agent
8
About 5% of the active ingredient
85% ethanol
Appropriate amount
/
[0073] In the above prescription, the particle size of the pellet core is 500-700 μm, of which the pellet core with a particle diameter of 500-600 μm accounts for 35%, and the talcum powder is 3000 mesh.
[0074] 2. Disposal of the first sustained-release pills: based on 1000 pills
[0075] material ...
Embodiment 3
[0082] The preparation of embodiment 3 venlafaxine hydrochloride sustained-release pellets
[0083] 1. Prescription of pills: 2000 pills (1000 pills for the first pill and 1000 pills for the second pill)
[0084] material
Prescription volume (g)
Proportion (%)
effect
200
/
Ball core
200
About 100% of the ball core
active ingredient
2
About 1% of active ingredient
85% ethanol
Appropriate amount
/
[0085] In the above prescription, the particle size of the ball core is 500-700 μm, and the ball core with a particle size of 500-600 μm accounts for 65%; the talcum powder is 3000 mesh.
[0086] 2. Disposal of the first sustained-release pills: based on 1000 pills
[0087] material
Prescription volume (g)
Proportion (%)
effect
first pill
195
/
/
Ethyl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com