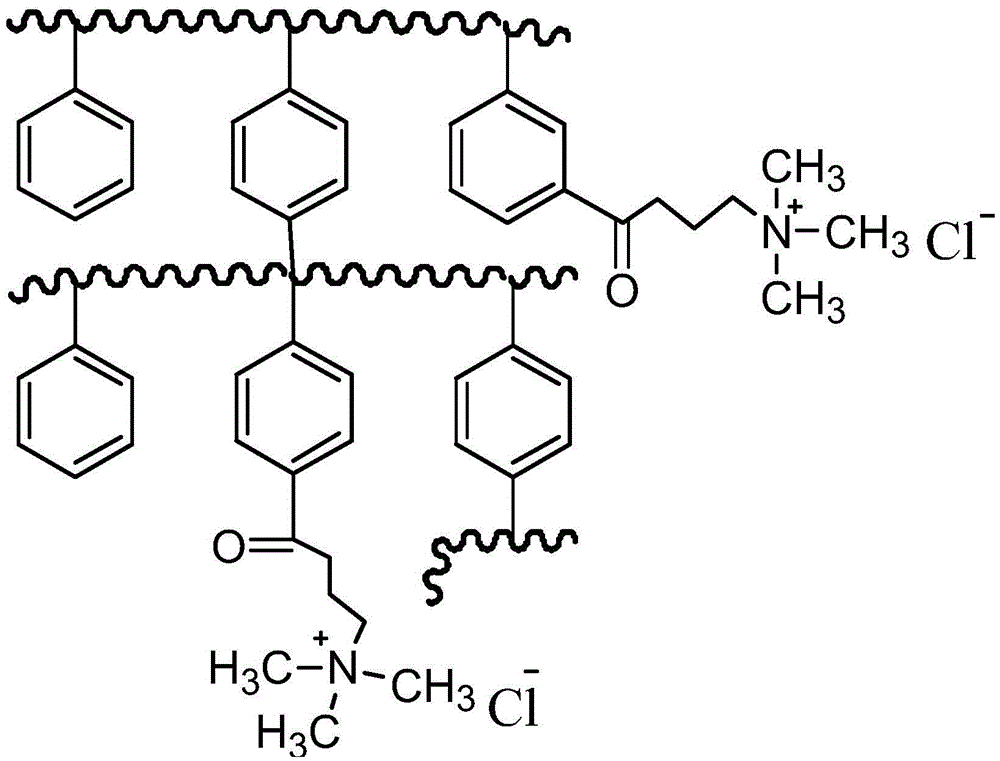
Adsorbent, preparation method thereof, and resin containing long-chain quaternary ammonium salt
A technology of adsorbent and quaternary ammonium salt, which is applied in the field of strong basic anion exchange resin, can solve the problems of decreased adsorption performance of adsorbent, discomfort reaction of related personnel, carcinogenic toxicity and other problems, and achieves the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
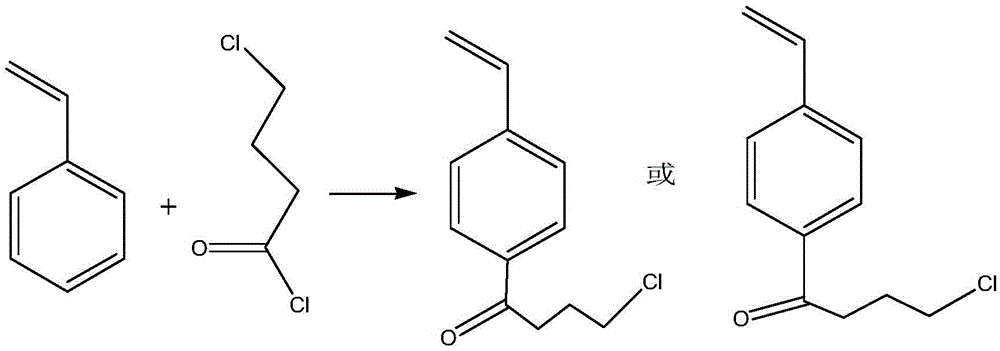
[0046] When preparing the adsorbent, first prepare 4-chlorobutyrylstyrene monomer, such as figure 2 Shown, the preparation process of 4-chlorobutyrylstyrene monomer is as follows:
[0047] First, add 100ml of chlorobutyryl chloride, 200ml of dichloroethane, and 1g of anhydrous ferric chloride into a 1L three-necked flask. Then slowly add 100ml of styrene dropwise at a rate of 1 drop / second to 3 drops / second under stirring conditions, while controlling the system temperature at 25±5°C, and keep the room temperature after the dropwise addition to continue the reaction for 5.5 hours. Then, after the reaction is completed, add an ice-water solution of hydrochloric acid to wash away ferric chloride in a liquid separation manner, and remove the solvent and raw materials not involved in the reaction by distillation under reduced pressure. Finally, it is separated and purified to obtain 4-chlorobutyrylstyrene monomer.
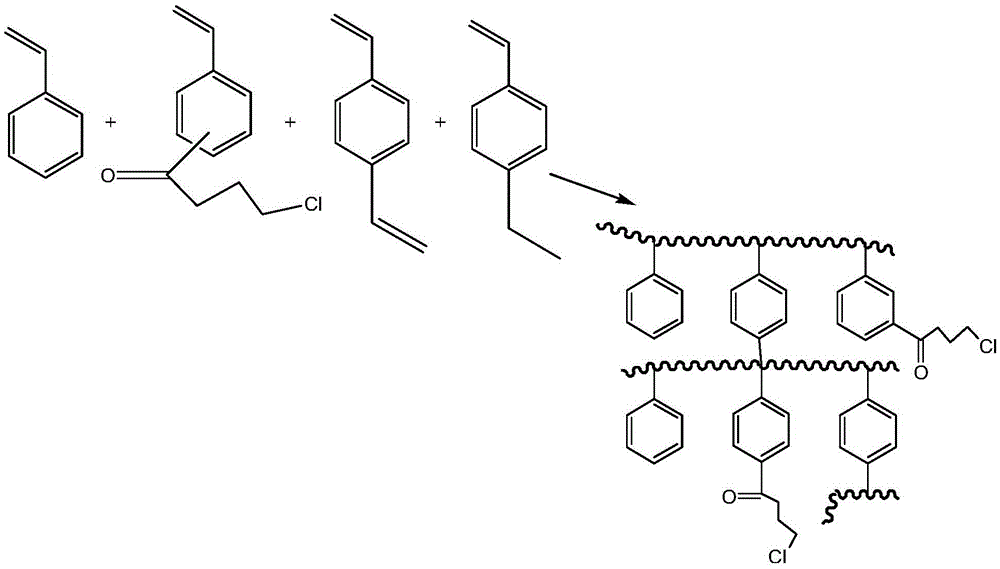
[0048] Such as image 3 Shown, the preparation process of pol...
Embodiment 2
[0056] The preparation process of the polystyrene-based macroporous resin matrix in this example is as follows: first, 800 ml of an aqueous solution containing 1.5 wt % gelatin and 3 wt % sodium chloride was added to a 2 L three-necked flask. Then add 10g of divinylbenzene, 8.2g of ethylstyrene, 61.8g of styrene, 20g of 4-chlorobutyrylstyrene, 125g of isooctyl alcohol, 25g of butyl acetate, and 1g of lauryl peroxide. The organic phase. Then, the temperature was raised to 68° C. for 5 hours for polymerization under mechanical stirring, and the temperature was raised to 80° C. for 10 hours to stop the reaction after curing. Then wash off the porogen iso-octanol with methanol, then wash with water until the washing solution is clear, filter with suction, and sieve. Finally, a resin with a particle size in the range of 0.3 mm to 0.6 mm is selected to obtain the polystyrene-based macroporous resin matrix containing 4-chlorobutyrylphenyl in this embodiment.
[0057] Other experime...
Embodiment 3
[0060] The preparation process of the polystyrene-based macroporous resin matrix in this example is as follows: first, 800 ml of an aqueous solution containing 1.5 wt % gelatin and 3 wt % sodium chloride was added to a 2 L three-necked flask. Then add 12g divinylbenzene, 9.8g ethylstyrene, 48.2g styrene, 30g 4-chlorobutyrylstyrene, 120g isooctyl alcohol, 40g butyl butyrate, 1g lauryl peroxide The organic phase. Then, the temperature was raised to 68° C. for 5 hours for polymerization under mechanical stirring, and the temperature was raised to 80° C. for 10 hours to stop the reaction after curing. Then wash off the porogen iso-octanol with methanol, then wash with water until the washing solution is clear, filter with suction, and sieve. Finally, a resin with a particle size in the range of 0.3 mm to 0.6 mm is selected to obtain the polystyrene-based macroporous resin matrix containing 4-chlorobutyrylphenyl in this embodiment.
[0061] Other experimental procedures and metho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com