Tumor targeted polypeptide, and preparation method and application thereof
A technology targeting peptides and tumor markers, applied in the field of medicinal chemistry, can solve the problems of insufficient interaction and poor targeting effect, and achieve the effect of non-immunogenicity, small molecular weight and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1 Construction of the polypeptide screening system of the present invention
[0063] 1) Experimental instruments and materials
[0064] N-methylmorpholine (NMM), piperidine, trifluoroacetic acid (TFA), dichloromethane (DCM), ninhydrin, vitamin C, phenol, tetramethyluronium hexafluorophosphate (HBTU), hexahydro Pyridine, triisopropylsilane (TIS), ethanedithiol (EDT), N,N dimethylformamide (DMF), anhydrous ether, resin, methanol, various Fmoc protected amino acids, PE-anti-HER1 Antibody, PE-anti-HER2 antibody, Cy5-Streptavidin (streptavidin), FITC-Streptavidin (streptavidin), Bio-AdembeadsStreptavidin (streptavidin-labeled magnetic nanospheres), peptide synthesis tube, Shaker, vacuum water pump, rotary evaporator, laser confocal microscope (ZEISSLSM710), the above reagents and materials were obtained from commercial sources.
[0065] 2) Synthesis of "one bead, one object" polypeptide library
[0066] The Fmoc solid-phase peptide synthesis method is used to s...
experiment example 1
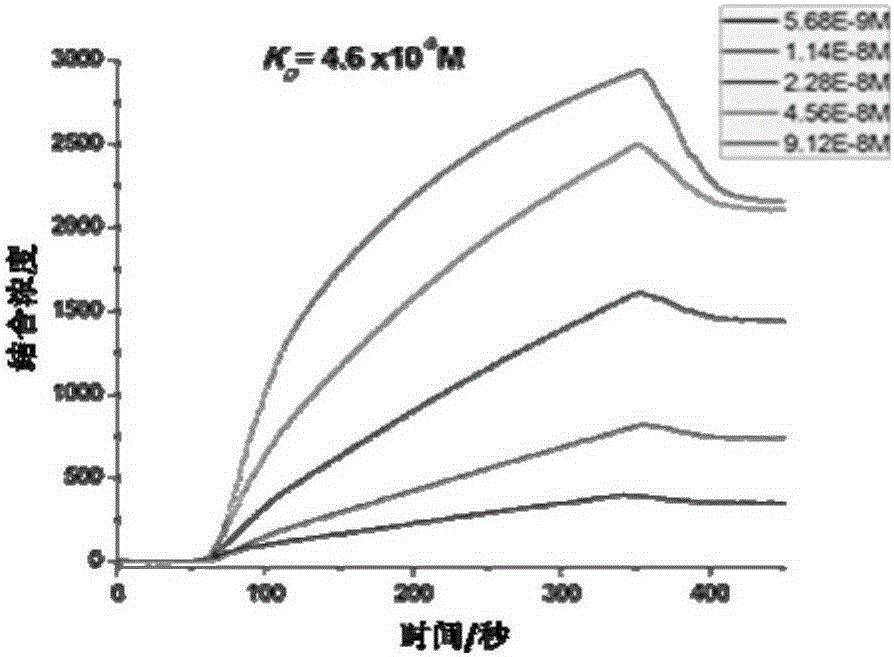
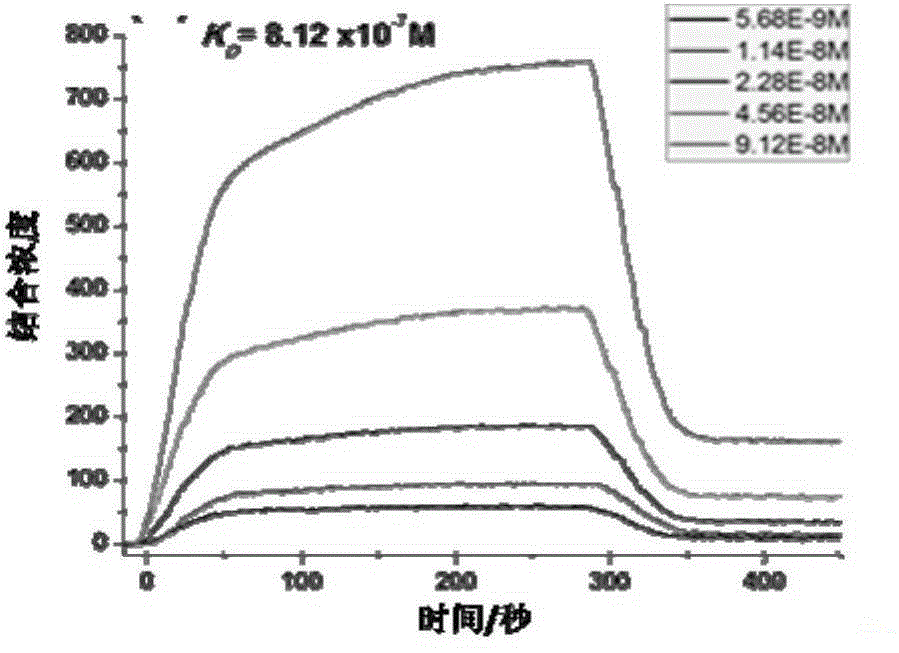
[0075] Experimental example 1 detects the affinity of SEQ ID NO.1-3 polypeptides and HER1 and HER2 proteins by surface plasmon resonance (SPRi) method
[0076] Spot 1 mg / mL of SEQ ID NO.1-3 polypeptide and 1×PBS on the chip, incubate overnight at 4°C under humid conditions, then wash with 10×PBS for 10 minutes, then wash with 1×PBS for 10 minutes, and finally wash with deionized water Wash twice, 10min each time, immerse in 1×PBS containing 5% milk, incubate overnight at 4°C, then wash with 10×PBS for 10min, 1×PBS for 10min, and finally wash twice with deionized water, each Each time for 10 minutes, blow dry with nitrogen, and install the chip on the machine (Plexera HT surface plasmon resonance imaging system).
[0077] The mobile phase was sequentially passed through 1×PBS, 2×PBS, 0.625 μg / mL, 1.25 μg / mL, 2.5 μg / mL, 5 μg / mL and 10 μg / mL of human HER1 and HER2 purified proteins, and the SPRi signal was recorded and analyzed.
[0078] As can be seen from Figure 2(a)-(b), th...
experiment example 2
[0079] Experimental example 2 Interaction of SEQ ID NO.1-3 with HER1 and HER2 high expression cells MDA-MB-468 and SKBR3 and normal cell 293A
[0080] Breast cancer cell lines MDA-MB-468 and SKBR3 (HER2 highly expressed) cells were cultured in DMEM containing 20% fetal bovine serum and RPMI1640 medium containing 10% fetal bovine serum, respectively, and normal human kidney fibroblasts 293A were cultured in In H-DMEM medium containing 10% fetal bovine serum, 1×10 5 / mL cell concentration into a round glass-bottom Petri dish (35mm), 37 ° C, 5% CO 2 After culturing in the cell incubator for 24 hours, discard the culture medium, add 50 μmol / L FITC-labeled H1P, H2P, and HMP polypeptides containing 1 μmol / L Hoechst33342 to the three types of cells, and incubate at 4°C for 0.5 hours in the dark, then discard the polypeptides respectively solution, and washed twice with pre-cooled 1×PBS, and added 5 μL PE-anti-HER1 antibody and PE-anti-HER2 antibody respectively. After incubating ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com