Dry suspension containing Arbidol and salt thereof as well as preparation method of dry suspension
A technology of arbidol hydrochloride and suspension, which is applied in the direction of antiviral agent and powder delivery, etc. It can solve the problems of arbidol raw material not being resistant to high temperature, failing to achieve drug curative effect, delaying drug release, etc., to achieve effective Conducive to industrialization and product quality control, improve bioavailability, and avoid exposure to high temperatures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
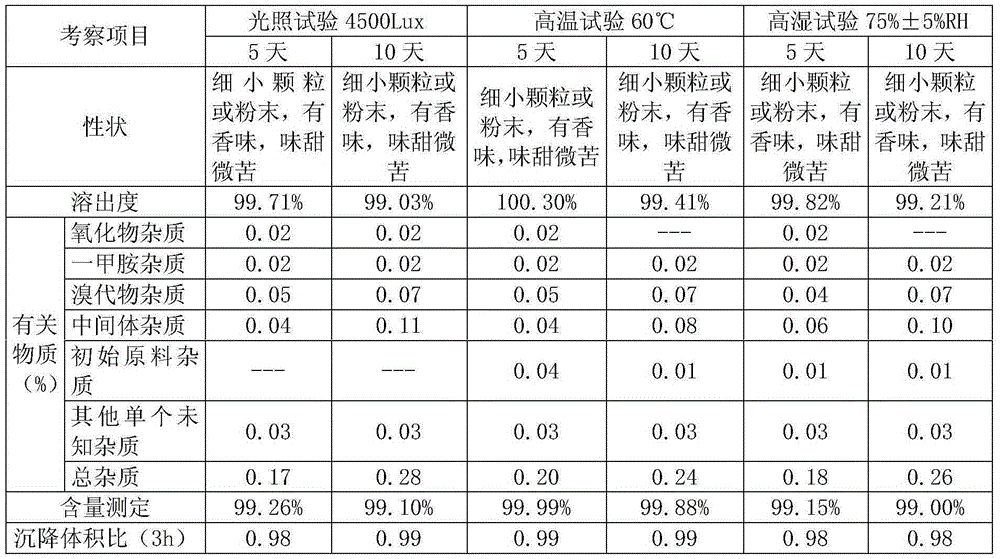
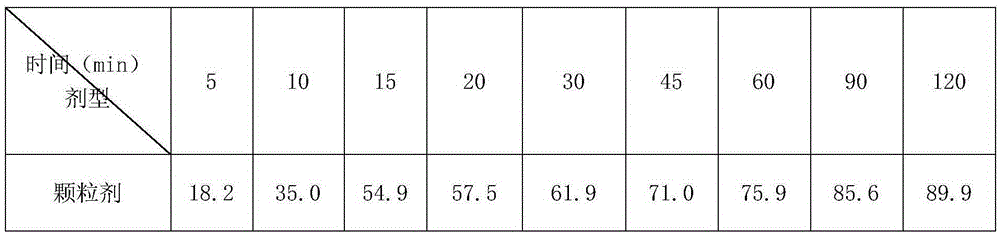
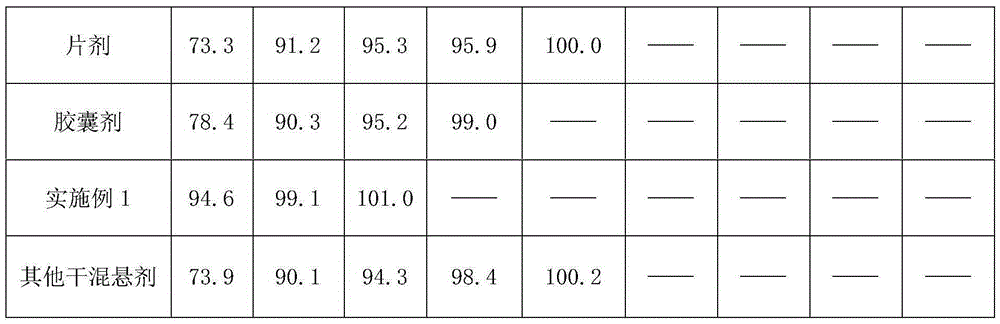
[0025] A kind of Arbidol and its salt dry suspension, take Arbidol hydrochloride 200g, hypromellose 40g, sucralose 2g, mannitol 200g, sucrose 1540g and peach essence 10g, 0.03% cochineal Appropriate amount of red pigment aqueous solution; control the particle size of Arbidol hydrochloride D90: 1-20 μm, crush sucralose and peach flavor through a 100-mesh sieve; crush sucrose and mannitol through a 80-mesh sieve; hypromellose through a 100-mesh sieve Sieve; fully mix sucrose and mannitol, add an appropriate amount of 0.03% cochineal pigment aqueous solution, make soft material, granulate with a 32 mesh sieve, dry at 50°C for 60 minutes, pass through a 28 mesh sieve for granulation, add arbidol hydrochloride, three Sucralose, hypromellose, and peach flavor are mixed evenly, and after passing the inspection, they are put into composite film bags to make 1000 bags of 2g each or 2000 bags of 1g each.
Embodiment 2
[0027]A kind of Arbidol and its salt dry suspension, get Arbidol hydrochloride 200g, Arabic gum 20g, hydroxypropyl cellulose 20g, mannitol 180g, aspartame 2.0g, sucrose 1560g and peach flavor 10g, appropriate amount of 0.03% lemon yellow pigment aqueous solution; Arbidol hydrochloride controlled particle size D90: 1-20 μm, sucrose, mannitol, crushed to 80 mesh; gum arabic, hydroxypropyl cellulose, aspartame, peach The essence is crushed separately and passed through a 100-mesh sieve; the sucrose and mannitol are fully mixed, and an appropriate amount of 0.03% lemon yellow pigment aqueous solution is added to make a soft material, granulated with a 32-mesh sieve, dried at 50°C for 90 minutes, passed through a 28-mesh sieve for granulation, and added with hydrochloric acid Bidol, aspartame, gum arabic, and peach essence are mixed evenly, and after passing the inspection, put them into a composite film bag to make 1000 bags of 2g each or 2000 bags of 1g each.
Embodiment 3
[0029] A kind of Arbidol and its salt dry suspension, get Arbidol hydrochloride 100g, hydroxypropyl cellulose 90g, xanthan gum 30g, sucrose 400g, mannitol 350g, aspartame 2.5g, lemon essence 10g, an appropriate amount of 0.03% lemon yellow pigment aqueous solution; Arbidol hydrochloride controlled particle size D90: 1 ~ 20μm, sucrose, mannitol crushed through 80 mesh; hydroxypropyl cellulose, xanthan gum, aspartame and lemon The essence is crushed separately and passed through a 100-mesh sieve; the sucrose and mannitol are fully mixed, and an appropriate amount of 0.03% lemon yellow pigment aqueous solution is added to make a soft material, granulated with a 32-mesh sieve, dried at 50°C for 100 minutes, passed through a 20-mesh sieve for granulation, and added with hydrochloric acid. Bidol, xanthan gum, hydroxypropyl cellulose, aspartame and lemon essence are mixed evenly. After passing the inspection, put them into a composite film bag to make 1000 bags, 2g per bag or 2000 bag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com