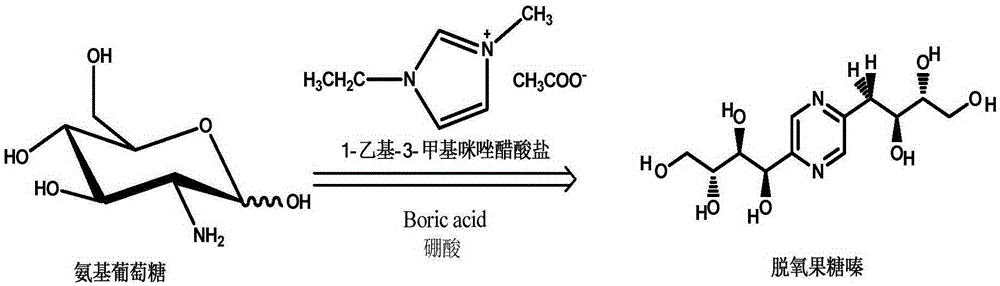
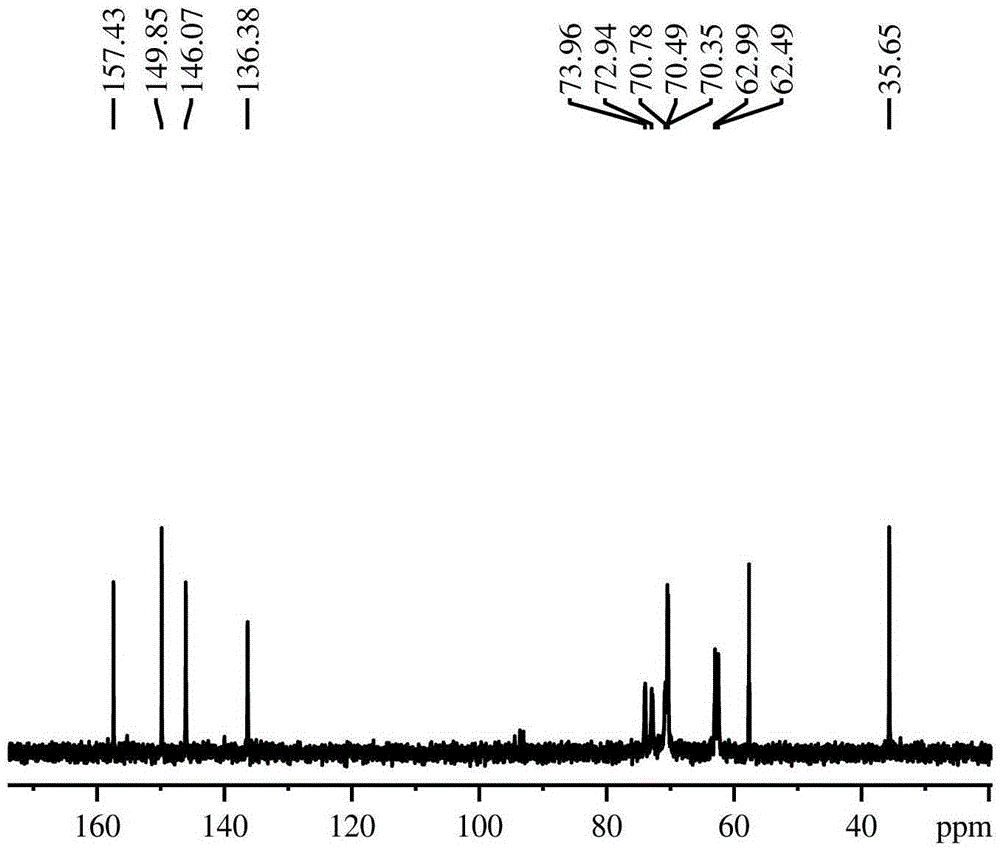
Method for preparing deoxyfructosazine through chitin biomass
A technology of deoxyfructozine and chitin, which is applied in the field of preparing high value-added nitrogen-containing heterocyclic compound deoxyfructozine, can solve the problems of complex steps, long reaction time, low yield, etc. The effect of stabilizing the temperature range and improving the reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) First prepare 1-ethyl-3-methyl-imidazolium acetate ionic liquid, add 0.1mol 1-ethyl-3-methylimidazolium bromide (19.1g) and 2.5mol Potassium acetate (245.3g), was dissolved by adding 160ml of isopropanol, and stirred at room temperature for 36h. Filter, remove insoluble matter, and spin evaporate the filtrate at 70°C for 8 hours to obtain a light yellow oil, dissolve it in 80ml of dichloromethane, add activated carbon powder and stir at room temperature for 17 hours, filter, wash with carbon chloride for 3 times, and combine the filtrates and spin evaporate The solvent was removed to obtain a tan oil, which was dried under vacuum at 70°C to obtain the target product. Take 10 g of 1-ethyl-3-methyl-imidazole acetate ionic liquid, add 10 g of D-glucosamine hydrochloride with a molecular weight of 215.5, add boric acid equimolar to D-glucosamine hydrochloride, add After mixing evenly in methyl sulfoxide, react at 25°C for 48 hours.
[0033] (2) After the reaction, at ...
Embodiment 2
[0035] (1) Prepare ionic liquid as in the method in Example 1, get 25g of 1-ethyl-3-methyl-imidazole acetate ionic liquid, add 50g molecular weight and be the D-glucosamine sulfate sodium salt of 282.2, add and raw material D-Glucosamine Sulfate Sodium Salt Equimolar sodium tetraborate was uniformly mixed in 100 ml of dimethyl sulfoxide, and reacted at 80° C. for 10 minutes.
[0036] (2) After the reaction, at room temperature, take 25 ml of the reaction product solution, add 50 ml of acetonitrile, extract five times, remove insoluble impurities, concentrate by rotary evaporation, and stand for crystallization to obtain the product deoxyfructozine. The extraction agent is recovered, and the imidazole ionic liquid is recovered and reused. The conversion rate of the reaction raw material D-glucosamine sulfate sodium salt is 100%, the purity of the crystallized product is more than 98%, and the molar yield of deoxyfructosine is 55%.
Embodiment 3
[0038] (1) The preparation method of ionic liquid is similar to the preparation method of 1-ethyl-3-methyl-imidazolium acetate ionic liquid, adds 1-butyl-3-methylimidazolium bromide and Potassium acetate reaction, the molar ratio of reactants and the reaction conditions are the same as in Example 1, and 1-butyl-3-methyl-imidazole acetate ionic liquid is prepared. Get 40g of 1-butyl-3-methyl-imidazole acetate ionic liquid, add 35g molecular weight and be 250,000 chitins, add the glacial acetic acid that molar weight is 250,000 chitins of raw material molecular weight, in 100ml Mix evenly in dimethyl sulfoxide, and react at 150°C for 6 hours.
[0039] (2) After the reaction, at room temperature, take 3 ml of the reaction product solution, add 15 ml of acetonitrile, extract twice to remove insoluble impurities, concentrate the filtrate by rotary evaporation, leave it for 15 hours, and crystallize to obtain the product. The conversion rate of the chitin with a molecular weight of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com