Organic micromolecular semiconductor material and synthesis method therefor and application thereof
A small molecule and semiconductor technology, applied in the field of linear oligothiophene organic small molecule semiconductor materials, can solve the problems of high price, complicated synthesis and purification process, slow development of electronic materials, etc., and achieve low cost, easy preparation, synthesis and purification The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] Another aspect of the present invention provides a method for synthesizing an organic small-molecule semiconductor material, comprising: providing a first compound comprising a diaromatic pentadiene-like π-conjugated unit and a second compound comprising an aromatic group, The organic small molecule semiconductor material is obtained through transition metal catalyzed condensation reaction.
[0040] Wherein, the first compound may have the following structural formula:
[0041]
[0042] Wherein, the second compound may have the following structural formula:
[0043]
[0044] Among them, X 1 、X 2 independently selected from O, S or Se, X 3 Selected from O, S, Se, NR 2 , C(R 2 ) 2 or Si(R 2 ) 2 , X 4 、X 5 independently selected from halogen atoms, Sn(R 4 ) 3 , BO(R 5 ) 2 Or a 5-7 membered ring substituted or unsubstituted boron ester containing -O-B-O-, and only one of X4 and X5 is a halogen atom, R 1It is selected from a straight chain or branched ch...
Embodiment 1
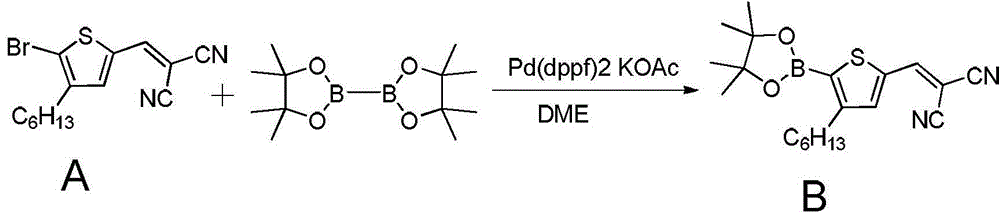
[0062] Embodiment 1 refers to figure 1 , the synthesis and preparation process of the organic small molecule semiconductor material can be:
[0063] Add raw material A (2.51g, 7.77mmol) into a 100ml single-necked round bottom flask, add pinacol diboronate (3.9g) and [1,1'-bis(diphenylphosphino)ferrocene] under nitrogen atmosphere Palladium dichloride (570mg), potassium acetate (2.28g), ethylene glycol dimethyl ether (55ml). Stir the reaction at 70° C. for 10-15 hours, wash with water after the reaction, separate the liquids, and combine the organic phases. The organic phase was dried over anhydrous sodium sulfate, and the organic solution was removed by rotary evaporation. The crude product was recrystallized from methanol to obtain the final product B (2.11 g), with a yield of 75%.
[0064] The characterization data for this product B are as follows: 1 HNMR (400MHz, CDCl 3 )δ=7.76(s,1H),7.66(s,1H),2.90–2.78(m,2H),1.56(dd,J=14.7,7.1Hz,2H),1.34(s,13H),1.32–1.27 (m, 6H), 0...
Embodiment 2
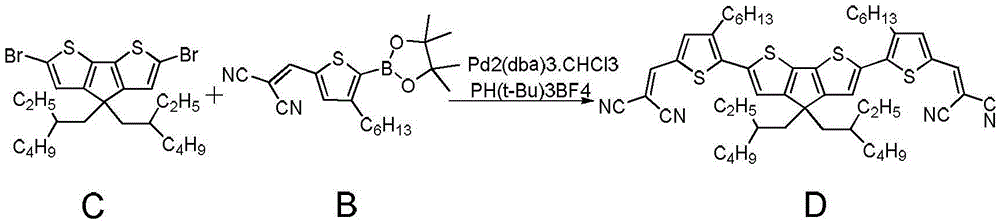
[0065] Embodiment 2 refers to figure 2 , the synthesis and preparation process of the organic small molecule semiconductor material can be:
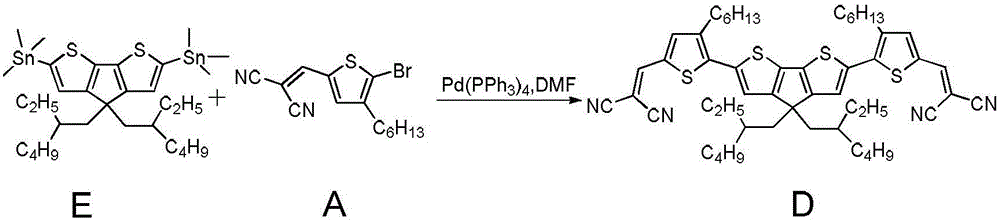
[0066] Add raw material B (50mg, 135μmol), raw material C (30mg, 54μmol) into a 25ml single-necked round bottom flask, tris(dibenzylideneacetone) dipalladium (7mg, 6.75μmol), tri-tert-butylphosphine tetrafluoroborate (3.9 mg, 13.5μmol) was added to the reactor under nitrogen protection, and fully degassed tetrahydrofuran (3ml) was added to the reactor, stirred to dissolve the raw material and catalyst, sodium carbonate solution (0.81ml, 1mol / L) was dropped into the reaction solution, and stirred After about 2 hours, after the reaction was completed, it was washed with water, separated, and the organic phases were combined. The organic phase was dried over anhydrous sodium sulfate, the organic solution was removed by rotary evaporation, and purified by thin-plate chromatography to obtain the final product D (47.9 mg) with a yield of 74%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com