Synthetic method for (S)-Virol A from water hemlock extract
A synthesis method and extract technology are applied in the field of asymmetric synthesis of hemlock extract-VirolA, which can solve the problems of difficulty in extraction and synthesis methods, decomposition and deterioration, and achieve cost reduction, simplification of reaction operations, efficient preparation process and environmental protection. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
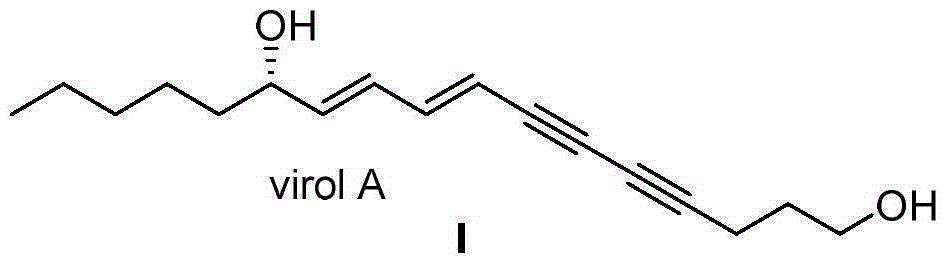
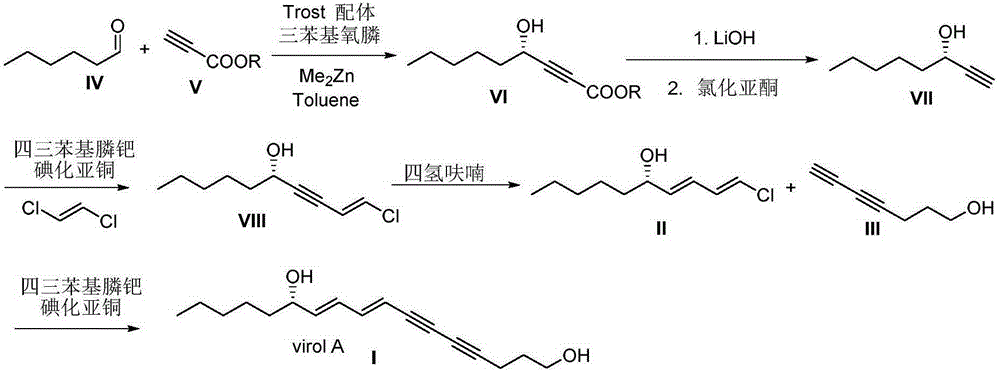
[0026] Synthesis of (S)-VirolA(I):
[0027] Under nitrogen protection, in a 10mL Shrek tube equipped with a magnetic stirrer, add cuprous iodide (57mg, 0.3mmol), tetrakistriphenylphosphine palladium (86mg, 0.75mmol), 4,6-diyne- 1-Heptanol (III) (282mg, 1.5mmol) and 3mL of benzene, stirred well. After reacting at room temperature for 3 minutes, (S,1E,3E)-1-chloro-1,3-diene-5-decanol (II) (283.5mg, 1.5mmol) and triethylamine (2mL, 15mmol) were added sequentially , react overnight at room temperature. The reaction was quenched by adding 3 mL of saturated ammonium chloride solution. The aqueous phase was extracted with ether, the obtained organic phase was dried with anhydrous sodium sulfate, and the crude product was obtained by distillation under reduced pressure. Finally, it was purified by silica gel column chromatography (petroleum ether / ethyl acetate=10 / 1) to obtain 332 mg of a colorless liquid with a yield of 85%. [α] D 20 =+15.7(c1.1, MeOH). 1 HNMR (300MHz, CDCl 3 ...
Embodiment 2
[0029] Synthesis of (S)-4-hydroxyl-2-yne nonanoic acid methyl ester (VI):
[0030]In a 100ml Shrek bottle under nitrogen protection, 1.2777g (2mmol, 20% equiv) of Trost ligand and 1.1131g (4mmol, 40% equiv) of triphenylphosphine oxide were sequentially added. Cool to 0°C, add methyl propiolate 0.90ml (10mmol, 1.0equiv) successively, anhydrous toluene 15ml, slowly add dimethyl zinc solution 25ml (1.2mol / l, 30mmol, 3equiv), after the dropwise addition , continue to react for 30min. Lower the reaction system to -20°C, slowly add 4.8ml (40mmol, 4.0equiv) of hexanal dissolved in 15ml of toluene with a syringe pump within three hours, after the dropwise addition, continue to react for 5h, and detect by thin-layer chromatography. After the reaction was completed, 10ml of water was added, filtered with suction, extracted with ethyl acetate, washed with sodium chloride, dried over anhydrous sodium sulfate, precipitated, purified by column chromatography (ethyl acetate / petroleum ether=...
Embodiment 3
[0032] Synthesis of (S)-1-octyn-3-ol (VII):
[0033] In a 250ml three-necked flask, sequentially add (S)-4-hydroxy-2-alkyne methyl ester (VI) 0.9211g (5mmol, 1eq), tetrahydrofuran 25ml, and slowly add 1MaqLiOH (25mmol, 5eq) dropwise. Reaction 1h. Add 1MaqNaHSO to the system 4 50ml, after the dropwise addition, separate the liquids, extract with ethyl acetate, dry over anhydrous sodium sulfate, and remove the solvent. The obtained oil was dissolved in 12ml of acetonitrile, CuCl (6mmol, 1.2eq) was added, and reacted at room temperature for 13h. Add 120ml of diethyl ether, wash with brine, dry over anhydrous sodium sulfate, desolventize, and purify by column chromatography (ethyl acetate / petroleum ether=20 / 1) to obtain light oily liquid (S)-1-octyn-3-ol (VII) 0.536g, yield 85%. 1 HNMR (300MHz, CDCl 3 )δ4.30(m,1H),3.10(s,1H),2.40(d,J=2.1Hz,1H),1.75–1.54(m,2H),1.46–1.33(m,2H),1.33–1.11 (m,4H),0.83(t,J=7.0Hz,3H). 13 CNMR (75MHz, CDCl 3 )δ84.82,72.31,61.71,37.15,31.03,24.33,2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com