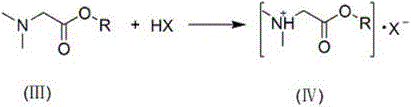Degradable gemini quaternary ammonium salt bactericide and preparation method thereof
A technology of geminiquaternary ammonium salts and fungicides, which is applied in the field of preparation of geminiquaternary ammonium salt fungicides, can solve the problems of no literature reports on geminiquaternary ammonium salts, no ability to kill fungi, and slow degradation of quaternary ammonium salts. High yield, strong bactericidal effect, strong adsorption and bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
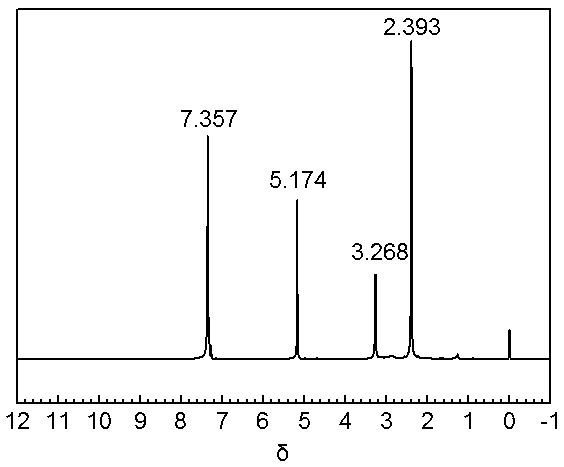
[0023] 1. Preparation of benzyl N,N-dimethylglycine: Weigh 10.12g (100mmol) of N,N-dimethylglycine, 12.98g benzyl alcohol (120mmol), 23.01g EDCI (120mmol) and 1.22g DMAP (10mmol) Place in 250mL containing 50mL, and react at room temperature for 24h. After the reaction, add an appropriate amount of saturated saline, extract with CH2Cl2, combine the organic phases, remove the solvent by rotary evaporation and then distill to obtain 13.53g of N,N-dimethylglycine benzyl ester ( 70%). Its NMR spectrum is attached figure 1 ;
[0024] 2. Preparation of N,N-dimethylglycine benzyl ester hydrochloride: Dissolve 6.76g (35mmol) N,N-dimethylglycine benzyl ester in 30mL ethanol solution, and add dry hydrogen chloride gas under stirring , until the precipitate was completely separated out, cooled, vacuum filtered, and dried to obtain 10.17g of N,N-dimethylglycine benzyl ester hydrochloride;
[0025] 3. Preparation of Gemini quaternary ammonium salt: 6.76g N,N-dimethylglycine benzyl ester,...
Embodiment 2
[0027] 1. Preparation of N,N-dimethylglycine octyl ester: Weigh 10.12g (100mmol) of N,N-dimethylglycine, 15.63g n-octanol (120mmol), 24.74gDCC (120mmol) and 1.22gDMAP (10mmol) ) was placed in 250mL containing 50mL, and reacted at room temperature for 24h. After the reaction, the insoluble matter was removed by filtration, washed with CH2Cl2, the organic phase was combined, the solvent was removed by rotary evaporation and then distilled to obtain 15.50g N,N-dimethylglycine n-octyl ester (72%);
[0028] 2. Preparation of N,N-dimethylglycine n-octyl hydrochloride: take 7.75g (36mmol) N,N-dimethylglycine n-octyl ester and dissolve it in 30mL ethanol solution, and pass it into the dry Hydrogen chloride gas until the precipitate is completely separated out, cooled, vacuum filtered, and dried to obtain 11.35g N,N-dimethylglycine n-octyl hydrochloride;
[0029] 3. the preparation of gemini quaternary ammonium salt: with above-mentioned prepared 7.75gN, N-dimethylglycine n-octyl este...
Embodiment 3
[0031] 1. Preparation of N,N-dimethylglycine decyl ester: Weigh 10.12g (100mmol) of N,N-dimethylglycine, 18.99g of n-decyl alcohol (120mmol), 23.01g of EDCI (120mmol) and 16.28g of HOBT (120mmol) ) was placed in 250mL containing 50mL, and reacted at room temperature for 24h. After the reaction was completed, it was washed with saturated brine, then extracted with CH2Cl2, the organic phases were combined, the solvent was removed by rotary evaporation and then distilled to obtain 16.55g of N,N-dimethylglycine decyl ester ( 68%);
[0032] 2. Preparation of N,N-dimethylglycine n-decyl ester hydrochloride: Dissolve 8.28g (39mmol) N,N-dimethylglycine n-decyl ester in 30mL ethanol solution, and pass it into dry Hydrogen chloride gas, until the precipitate is completely separated out, cooled, vacuum filtered, and dried to obtain 12.18g N,N-dimethylglycine n-decyl ester hydrochloride;
[0033]3. Preparation of Gemini quaternary ammonium salt: 8.28g N, N-dimethylglycine n-decyl ester, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com