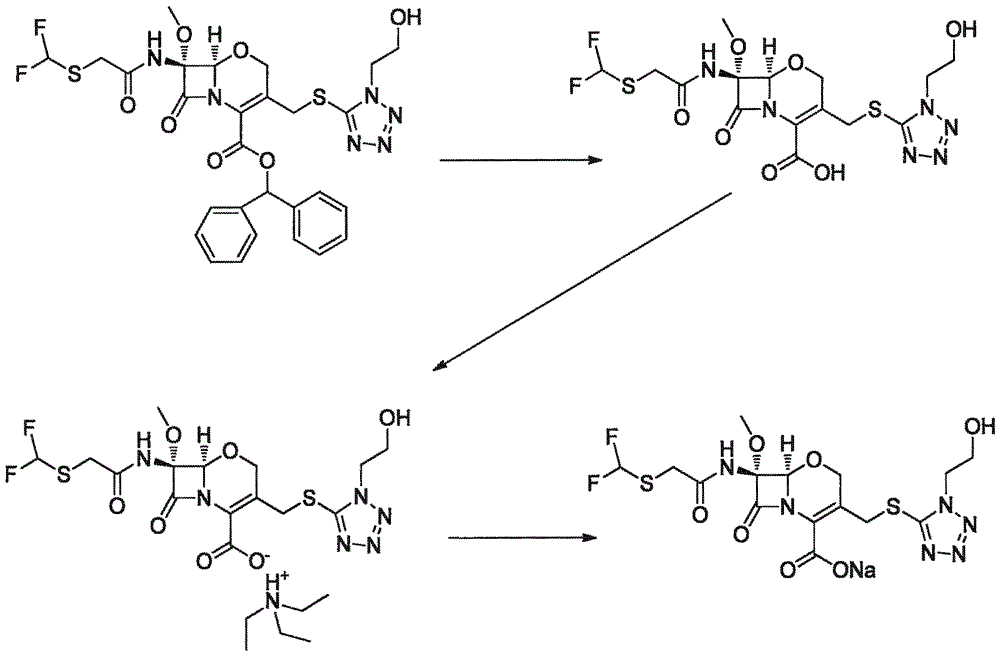
Synthesis method of flomoxef sodium
A technology of sodium fluoxetine and a synthesis method, which is applied in the field of synthesis and preparation technology of high-purity sodium fluoxafen, can solve the problems of harsh conditions, high energy consumption, inability to be dried and removed, etc., and achieves easy industrialization and low energy consumption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1) Synthesis of Fluoxetal
[0025] Put 50g FF-03 and 250ml m-cresol into the reaction bottle, and react at 65-70°C for 2-2.5h (pay attention to monitor the end point of the reaction). Fluoxetal solution system;
[0026] Cool to 0-5°C after the reaction, add 750ml ether and 200ml 20% NaCl aqueous solution, shake well, separate the aqueous layer W1, add 200ml 20% NaCl aqueous solution to the organic layer O1, separate the aqueous layer W2, combine W1 and the aqueous solution of W2; add 750ml of ethyl acetate to the aqueous solution, stir and lower the pH to 1-3, extract, and take the ethyl acetate layer. Add anhydrous MgSO 4 Appropriate amount, stir and dehydrate for 30-60 minutes, filter with suction, and transfer the filtrate into a reaction flask.
[0027] 2) Synthesis of Fluoxetin Triethylamine Salt
[0028] Drop the above reaction liquid to cool down to -15~-20°C, add triethylamine dropwise, precipitate solid, stir for 1-2h, filter, and dry under reduced pressure...
Embodiment 2
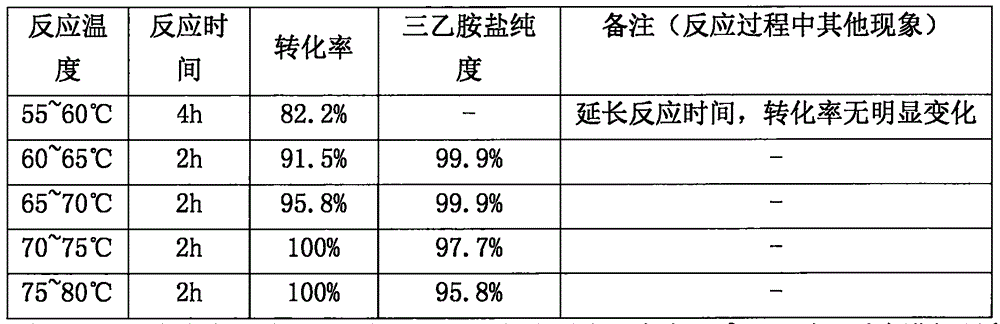
[0031] The influence that embodiment 2 step (1) temperature of reaction is selected on reaction carries out
[0032] 1. Put 50gFF-03 and 250ml m-cresol into the reaction bottle, and react at a certain reaction temperature (pay attention to monitoring the reaction end point). Fluoxetal solution system;
[0033] Cool to 0-5°C after the reaction, add 750ml ether and 200ml 20% NaCl aqueous solution, shake well, separate the aqueous layer W1, add 200ml 20% NaCl aqueous solution to the organic layer O1, separate the aqueous layer W2, combine W1 and the aqueous solution of W2; add 750ml of ethyl acetate to the aqueous solution, stir and lower the pH to 1-3, extract, and take the ethyl acetate layer. Add anhydrous MgSO 4 Appropriate amount, stirred and dehydrated for 30-60 minutes, filtered with suction, and transferred the filtrate into a reaction bottle. The results are shown in Table 1.
[0034] Table 1
[0035]
[0036] Conclusion: From the experimental results in the above...
Embodiment 3
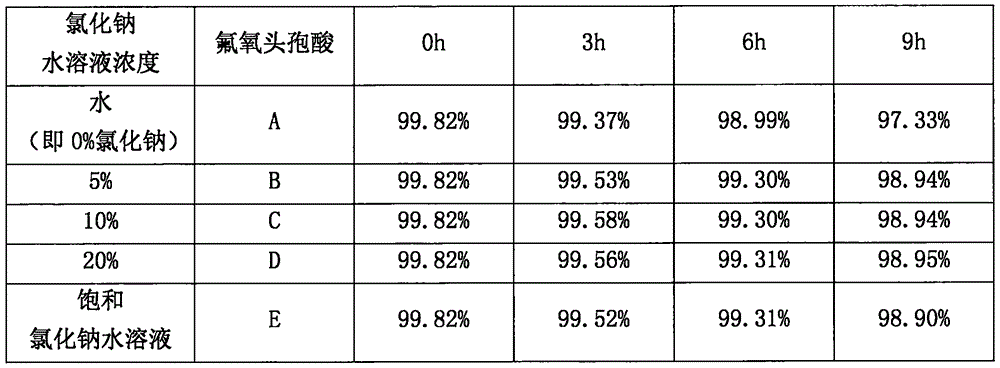
[0037] In the embodiment 3 step (1), the sodium chloride concentration selection is carried out on the influence of reaction
[0038] [Reaction] Put 50g FF-03 and 150ml m-cresol into the reaction bottle, and react at 65-70°C for 2-2.5 hours (pay attention to monitoring the reaction end point). Defloxacin solution system
[0039][Post-processing] After the reaction, cool to 0-5°C, divide the obtained fluoxetal solution into 5 parts, add 750ml of diethyl ether and 200ml of NaCl aqueous solution with different concentrations, shake well, separate the water layer W1, and the organic layer Add 200ml of a certain concentration of NaCl aqueous solution to O1, separate the aqueous layer W2, combine the aqueous solutions of W1 and W2; add 750ml of ethyl acetate to the aqueous solution, stir and lower the pH to 1-3, extract, and take the ethyl acetate layer. Add anhydrous MgSO 4 Appropriate amount, stirred and dehydrated for 30-60 minutes, filtered, and the filtrate was transferred to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com