Fluorescence identification dye based on nile blue matrix and preparing method and application thereof
A fluorescent recognition and Nile blue technology, applied in the field of fluorescent recognition dyes, can solve the problems of expensive medical treatment, real-time monitoring, and difficult cancer cure, and achieve low biomass background fluorescence, good photostability, and good application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] Another aspect of the present invention provides a class of preparation methods based on Nile blue parent fluorescent recognition dyes, said method comprising the steps of:
[0048] (1) 6-hydroxyl-2-naphthyl methyl ester reacts with a halogenated reagent in a molar ratio of 1:1~2 to prepare the compound of formula II:
[0049]
[0050] The reaction time is 6-36 hours, the reaction temperature is 10-80°C, and the reaction solvent is dichloromethane, ethanol, dimethylformamide, methanol, ethyl acetate or a mixture thereof;
[0051] In a preferred embodiment, the molar ratio of 6-hydroxy-2-naphthylmethyl ester to halogenated reagent is 1:1-1.5, the reaction time is 10-30h, the reaction temperature is 20-60°C, and the reaction solvent is dimethyl Formamide, ethanol, ethyl acetate or mixtures thereof;
[0052] In a more preferred embodiment, the molar ratio of 6-hydroxy-2-naphthylmethyl ester to halogenated reagent is 1:1-1.3, the reaction time is 20-30h, the reaction te...
Embodiment 1
[0080] Synthesis of fluorescent probe compound N1:
[0081]
[0082] (1) Synthesis of Intermediate 2
[0083] Dissolve 6-hydroxy-2-naphthylmethyl ester (10mmol) and NBS (12mmol) in 10ml of dimethylformamide respectively, and add the dimethylformamide solution of 6-hydroxy-2-naphthylmethyl ester into the round bottom flask , NBS was added dropwise into the round bottom flask using a constant pressure dropping funnel. During the reaction process, the reaction temperature was controlled at 28-32° C., the reaction system was filled with nitrogen for protection, and magnetic stirring was accelerated, and the reaction lasted for 24 hours. After the reaction was completed, the reaction solution was poured into ice water, and after standing still, a white precipitate was precipitated, which was dried by suction filtration and separated by chromatographic column to obtain intermediate 1 (60.5%) as a white solid.
[0084] (2) Synthesis of Intermediate 2
[0085] Dissolve Intermedi...
Embodiment 2
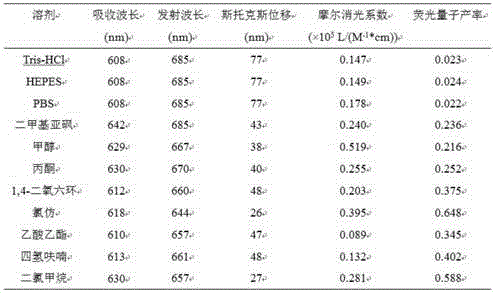
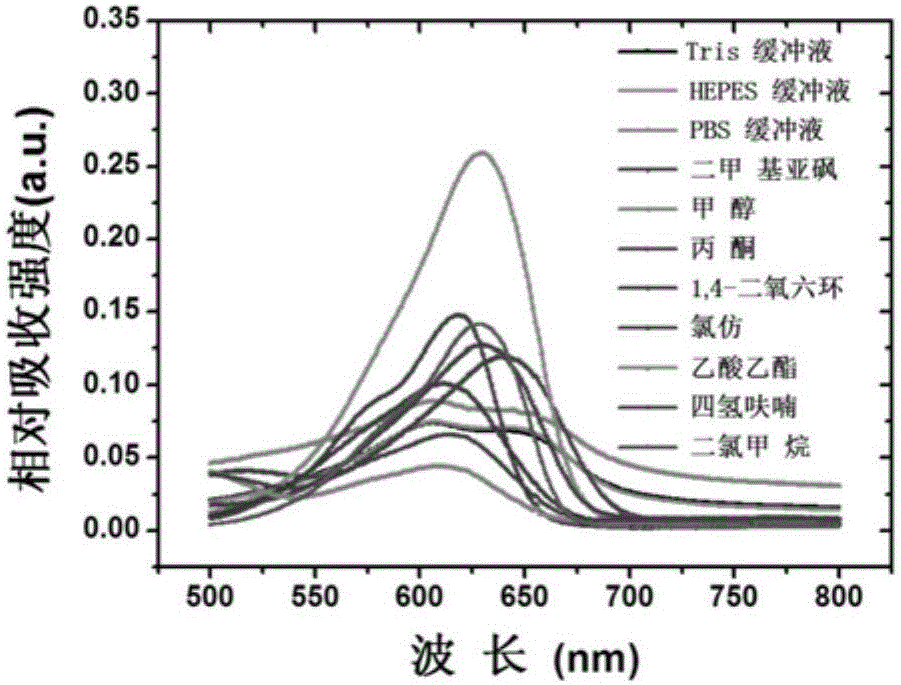
[0090] Photophysical Properties of Fluorescent Recognition Dye N1
[0091] Use the fluorescent recognition dye N1 prepared in Example 1 to add Tris-HCl buffer, PBS buffer, HEPES buffer, dimethyl sulfoxide, methanol, acetone, 1,4-dioxane, chloroform, acetic acid Ethyl ester, tetrahydrofuran, test ultraviolet absorption spectrum in dichloromethane ( Figure 2a ), fluorescence emission spectrum ( Figure 2b ), molar extinction coefficient and fluorescence quantum yield ( Figure 2c ), the dye concentration was 5 μM. The instruments used were AgIIlent8453 UV spectrophotometer and AgIIlentCaryEclIIpse fluorescence spectrophotometer. Figure 2 shows that the maximum absorption and emission wavelength of the fluorescent recognition dye N1 is greater than 630nm in the infrared region, has good photophysical properties, and is suitable for biological imaging applications.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com