A supramolecular hydrogel drug and gene dual carrier material and preparation method thereof
A supramolecular hydrogel and carrier material technology, which is applied in the field of supramolecular hydrogel drug and gene dual carrier materials and its preparation, achieves the effects of mild conditions, convenient and quick preparation method, low concentration and temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
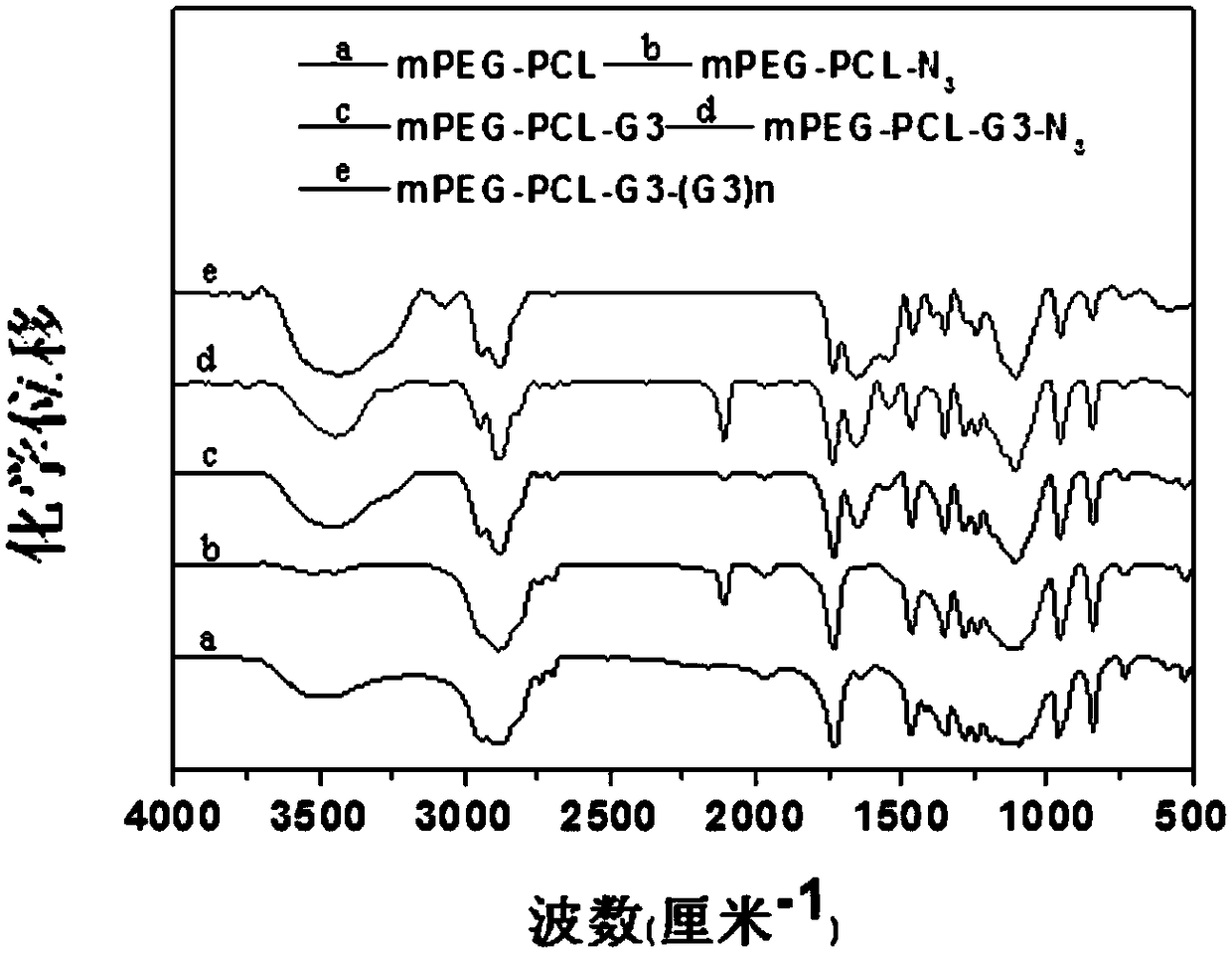
[0041] (1) polyethylene glycol-polycaprolactone-dendritic polylysine (mPEG-PCL-G 3 -(G 3 ) 3~8 ) Synthesis of multi-block polymers:
[0042] a. Lysine Lys(boc) protected by double Boc(N,N'-di-tert-butoxycarbonyl) 2 Dissolve in N,N-dimethylformamide, add propargylamine; Boc-protected lysine Lys(boc) 2 The molar ratio of propargylamine to propargylamine is 1:1.2; the system is placed in an ice-water bath and nitrogen protection is passed through at the same time; then 1-hydroxybenzotriazole (HOBt) and benzotriazole-N,N are added to the system ,N',N'-tetramethyluronium hexafluorophosphate (HBTU), the 1-hydroxybenzotriazole (HOBt) and benzotriazole-N,N,N',N'-tetramethyl The ratio of urea hexafluorophosphate (HBTU) was 1:1; after 24 hours at room temperature, the mixed system was diluted with 200 milliliters of ethyl acetate, and then saturated sodium bicarbonate, sodium bisulfate, sodium bicarbonate and Sodium chloride washing; The consumption of described sodium bicarbonate ...
Embodiment 2
[0051] (1) polyethylene glycol-polycaprolactone-dendritic polylysine (mPEG-PCL-G 3 -(G 3 ) 3~8 ) Synthesis of multi-block polymers:
[0052] a. Lysine Lys(boc) protected by double Boc(N,N'-di-tert-butoxycarbonyl) 2 Dissolve in N,N-dimethylformamide, add propargylamine; Boc-protected lysine Lys(boc) 2 The molar ratio of propargylamine to propargylamine is 1:1.2; the system is placed in an ice-water bath and nitrogen protection is passed through at the same time; then 1-hydroxybenzotriazole (HOBt) and benzotriazole-N,N are added to the system ,N',N'-tetramethyluronium hexafluorophosphate (HBTU), the 1-hydroxybenzotriazole (HOBt) and benzotriazole-N,N,N',N'-tetramethyl The ratio of urea hexafluorophosphate (HBTU) was 1:1; after 48 hours at room temperature, the mixed system was diluted with 200 milliliters of ethyl acetate, and then saturated sodium bicarbonate, sodium bisulfate, sodium bicarbonate and Sodium chloride washing; The consumption of described sodium bicarbonate ...
Embodiment 3
[0057] (1) polyethylene glycol-polycaprolactone-dendritic polylysine (mPEG-PCL-G 3 -(G 3 ) 3~8 ) The synthesis of multi-block polymer is the same as in Example 1
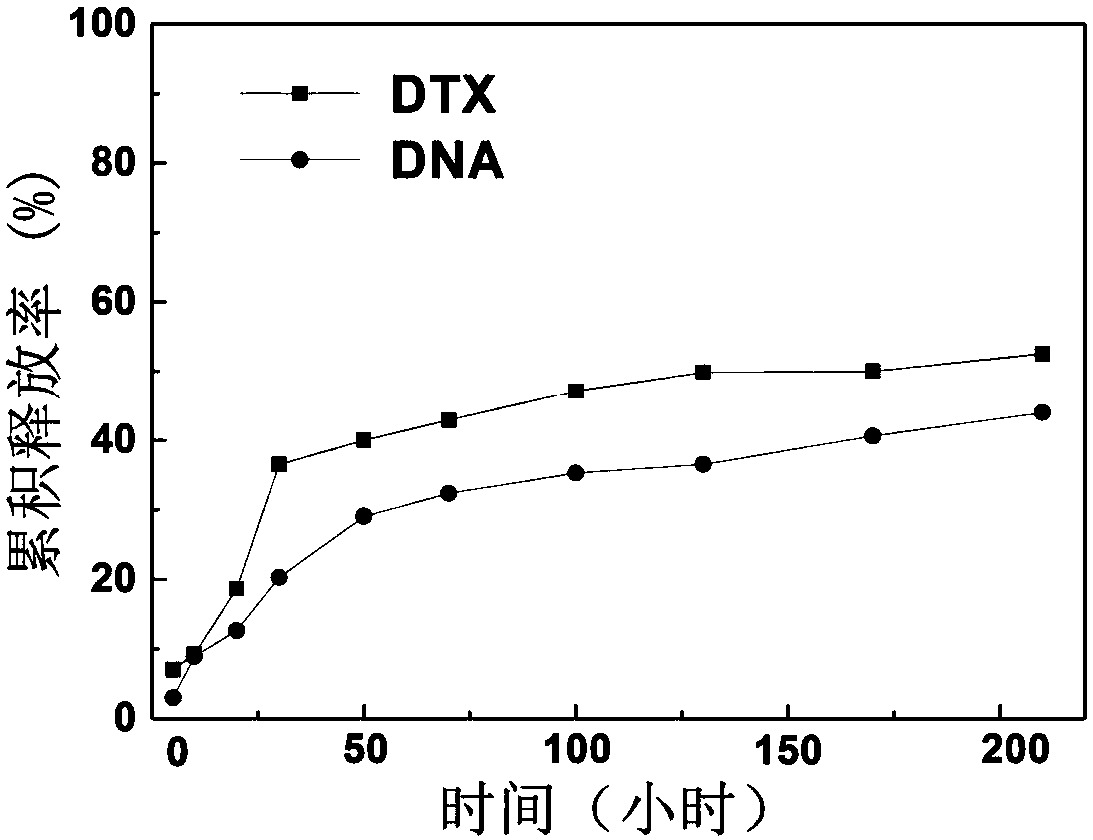
[0058] (2) At room temperature at 25°C, the polyethylene glycol-polycaprolactone-dendritic polylysine multi-block polymer A obtained in step (1) was formulated into 1 mL with a mass volume percentage concentration of 10% ( w / v) aqueous solution, under ice-bath conditions, add docetaxel acetone solution with a concentration of 2mg / ml therein, and stir magnetically at 37°C for 36 hours; mPEG-PCL-G 3 -(G 3 ) 3~8 / DTX complex;
[0059] (3) mPEG-PCL-G obtained in step (1) 3 -(G 3) 3~8 Dissolve in pure water to prepare a 1 mg / ml solution, filter and sterilize through a 0.2 μm filter head, mix with the DNA solution according to the N / P value of 2:1-80:1, and let it stand at room temperature for 20 minutes. get mPEG-PCL-G 3 -(G 3 ) 3~8 The polymer / nucleic acid complex solution of / DNA; With mPEG-PCL-G in step (2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com