Preparation method for chain extender used for structurally antibacterial polyurethane
A technology of polyurethane and chain extender is applied in the field of preparation of chain extender for structural antibacterial polyurethane, which can solve the problems of tissue cell toxicity, lack of biological selectivity, lack of long-term effect, etc. Reduced toxicity, wide application effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
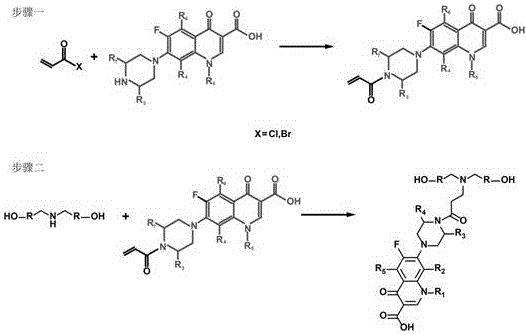
[0024] (1) Vinylation of ciprofloxacin: Mix 10 parts of ciprofloxacin, 4 parts of triethylamine and 300 parts of dichloromethane evenly, stir at 0°C for 30 minutes, then add dropwise under continuous stirring and nitrogen protection 4 parts of acryloyl chloride, after the dropwise addition, the temperature was raised to 20°C for 1 hour reaction; after the reaction, the above mixture was poured into n-hexane, and the precipitate was repeatedly washed with water and dried to obtain vinylated ciprofloxacin;
[0025] (2) Dihydroxy functional modification of vinylated ciprofloxacin: Dissolve 3 parts of vinylated ciprofloxacin, 1 part of diethanolamine and 0.5 part of triethylamine in 200 parts of methanol, and react at 40°C for 6 hours; after the reaction, methanol was distilled off under reduced pressure to obtain a yellow sticky solid, washed with anhydrous acetone, and the remaining solid was dried to obtain a dihydroxy chain extender containing ciprofloxacin side groups.
[002...
Embodiment 2
[0028] (1) Enoxacin vinylation: Mix 12 parts of enoxacin, 5 parts of pyridine and 360 parts of tetrahydrofuran evenly, stir at 2°C for 40 minutes, then add 5 parts of acryloyl chloride dropwise under constant stirring and nitrogen protection , after the dropwise addition was completed, the temperature was raised to 25°C to react for 1.5 hours; after the reaction was completed, the above mixture was poured into cyclohexane, and the precipitate was repeatedly washed with water and dried to obtain vinylated enoxacin;
[0029] (2) Dihydroxy functional modification of vinylated enoxacin: Dissolve 30 parts of vinylated enoxacin, 10 parts of dibutanolamine and 6 parts of pyridine in 300 parts of ethanol, react at 45 °C for 8 Hours; after the reaction, ethanol was distilled off under reduced pressure to obtain a yellow sticky solid, washed with anhydrous acetone, and the remaining solid was dried to obtain a dihydroxyl chain extender containing enoxacin side groups.
[0030] The above...
Embodiment 3
[0032] (1) Norfloxacin vinylation: 20 parts of norfloxacin, N,N -Mix 8 parts of diisopropylethylamine and 500 parts of ethyl acetate evenly, stir at 5°C for 60 minutes, then add 8 parts of acryloyl chloride dropwise under continuous stirring and nitrogen protection, and raise the temperature to 35°C to react for 2.5 hours; after the reaction is completed, the above mixture is poured into n-heptane, and the precipitate is repeatedly washed with water and dried to obtain vinylated norfloxacin;
[0033] (2) Dihydroxyl functional modification of vinylated norfloxacin: 300 parts of vinylated norfloxacin, 100 parts of diisopropanolamine and 18 parts of potassium carbonate were dissolved in 800 parts of chloroform, at 60°C React for 24 hours; after the reaction, chloroform was distilled off under reduced pressure to obtain a yellow sticky solid, washed with anhydrous acetone, and the remaining solid was dried to obtain a dihydroxyl chain extender containing norfloxacin side groups. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com