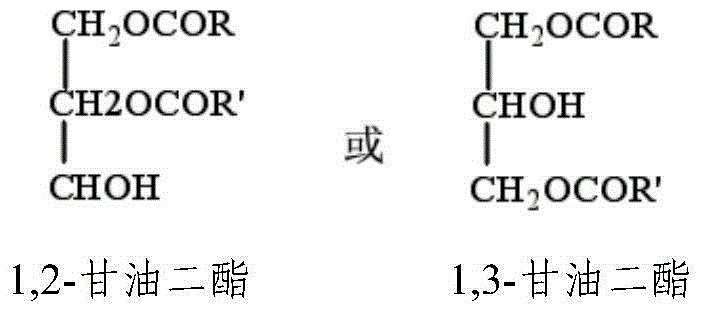
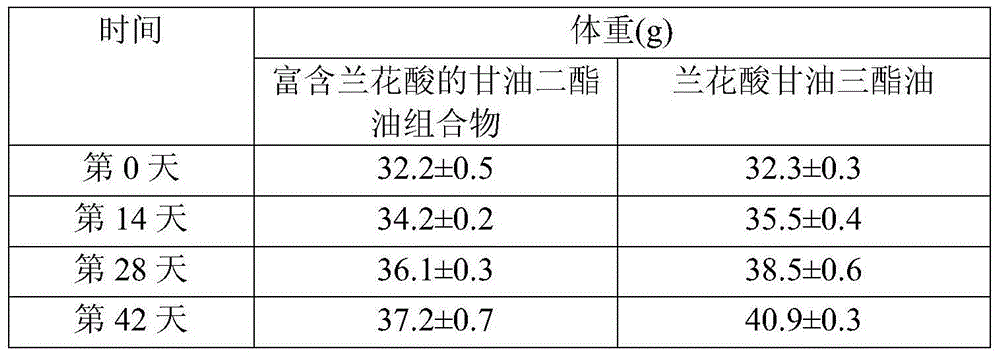
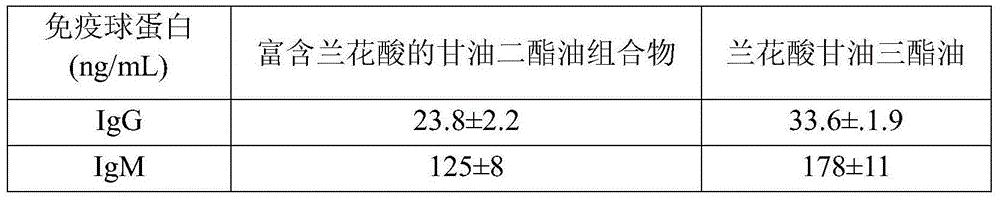
Diacylglycerol oil composition rich in orchid acid and preparation method and application of composition
A technology of diglyceride oil and diglyceride, which is applied in the field of applied chemistry, can solve the problems of increased oxidation, high price of lipase, complicated technological process, etc., and achieves the effects of improving blood lipids, simple preparation process and broad application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Collect jacaranda seeds, dry them in an oven at 40°C, and crush them to obtain 1000 g of dry powder. Reflux extraction with 5 times the volume of petroleum ether at a temperature lower than 60°C for 6 hours to obtain a green triglyceride crude extract with an oil yield of 28.2 %. Take 50 mL of crude triglyceride extract, add 200 mL of 2M 90% potassium hydroxide ethanol solution, saponify at 60°C under nitrogen protection for 2 hours, add 50 mL of water, then add 50 mL of petroleum ether for three extractions to remove non-soap compounds, and then add 6M hydrochloric acid After neutralizing and acidifying 100mL, extract the upper layer with petroleum ether to obtain a petroleum ether solution, and then obtain a crude cyanic acid extract after vacuum rotary evaporation. The crude extract of orchid acid was dissolved in ethanol, crystallized at 4°C under normal pressure for 8 hours to remove saturated fatty acids, and the obtained ethanol solution was further crystallized ...
Embodiment 2
[0032] Collect jacaranda seeds, dry them naturally under the sun, and crush them to obtain 1000 g of dry powder with a particle size of 0.4 to 0.5 mm. Under the conditions of 30 MPa and 40 ° C, they are extracted with supercritical CO2 (HA221-50 produced by Huaan Supercritical Extraction Unit, Nantong City, Jiangsu Province). -06 type supercritical extraction device) for 2 hours, the green triglyceride crude extract (crude oil) was obtained at a separation pressure of 15MPa and a separation temperature of 50°C, with an oil yield of 30.2%; the crude oil was degummed, deacidified, and decolorized , Deodorization, dewaxing and refining to obtain triglycerides. Take 50 mL of crude triglyceride extract, add 200 mL of 2M 90% potassium hydroxide ethanol solution, saponify at 60°C under nitrogen protection for 2 hours, add 50 mL of water, then add 50 mL of petroleum ether for three extractions to remove non-soap compounds, and then add 6M hydrochloric acid After neutralizing and acidi...
Embodiment 3
[0036] Collect jacaranda seeds, dry them in an oven at 40°C, and crush them to obtain 1000 g of dry powder. Extract them with 5 times the volume of a mixed solution of chloroform and methanol (volume ratio: 1:1) at room temperature for 24 hours to obtain green orchid triglycerides. The crude ester extract has an oil yield of 29.7%. Take 100mL of crude triglyceride extract, add 400mL of 2M 90% sodium hydroxide ethanol solution, saponify at 60°C under nitrogen protection for 2h, add 1000mL of water, then add 100mL of petroleum ether for three extractions to remove non-soap compounds, and then add 6M hydrochloric acid After neutralizing and acidifying 200mL, extract the upper layer with petroleum ether to obtain a petroleum ether solution. The crude extract of orchid acid was crystallized at 4°C under normal pressure for 10 hours to remove saturated fatty acids, and then crystallized at minus 40°C under normal pressure for 12 hours. Repeat 4 times Freeze crystallization to obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com