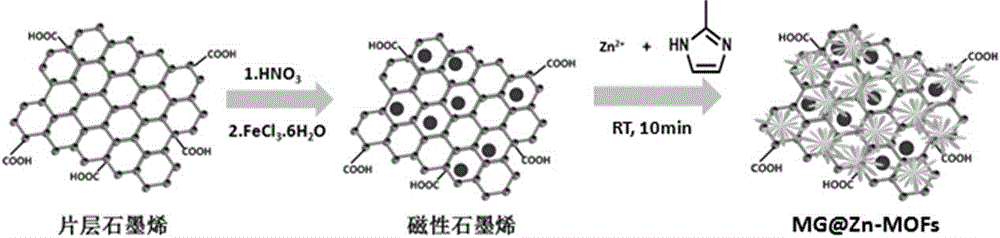
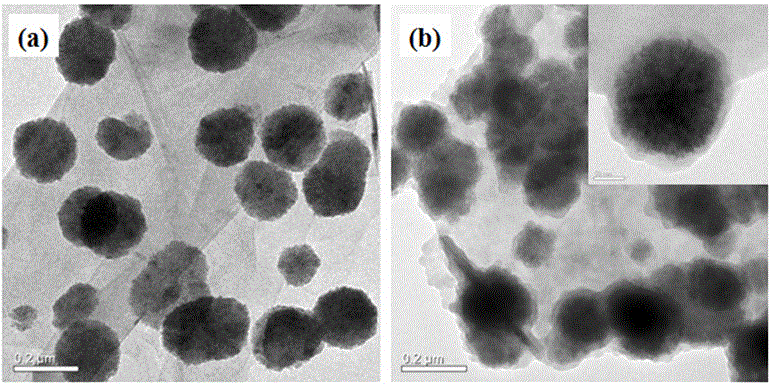
Synthetic method and application of metal organic skeleton-modified magnetic grapheme composite material
A metal-organic framework and magnetic graphene technology, applied in the field of nanomaterials, can solve the problems of weak ionization ability and low abundance of glycosylated proteins/peptides, and achieve good hydrophilic pore structure, high repetition rate, high reliability and high reliability. high noise effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: A synthetic method of a metal-organic framework-modified magnetic graphene composite.
[0032] (1) Disperse 400mg of graphene into 60mL of concentrated nitric acid, and reflux in a water bath at 60°C for 8 hours;
[0033] (2) centrifuging and separating the product obtained in step (1), washing it with deionized water until the solution is neutral, drying it in vacuum at 50°C, placing it for storage, and obtaining acidified graphene;
[0034] (3) 405mg FeCl 3 ·6H 2 O was dissolved in 40mL ethylene glycol solution, and stirred to obtain a yellow transparent solution;
[0035] (4) Disperse 150 mg of the product obtained in step (2) in the solution obtained in step (3), and sonicate for 1.0 hour;
[0036] (5) Under ultrasonic stirring, add 150 mg of trisodium citrate, 1.8 g of sodium acetate and 1.0 g of polyethylene glycol (20000) into the mixed solution obtained in step (4), and fully stir for 1.0 hour;
[0037](6) Transfer the mixed solution obtained in s...
Embodiment 2
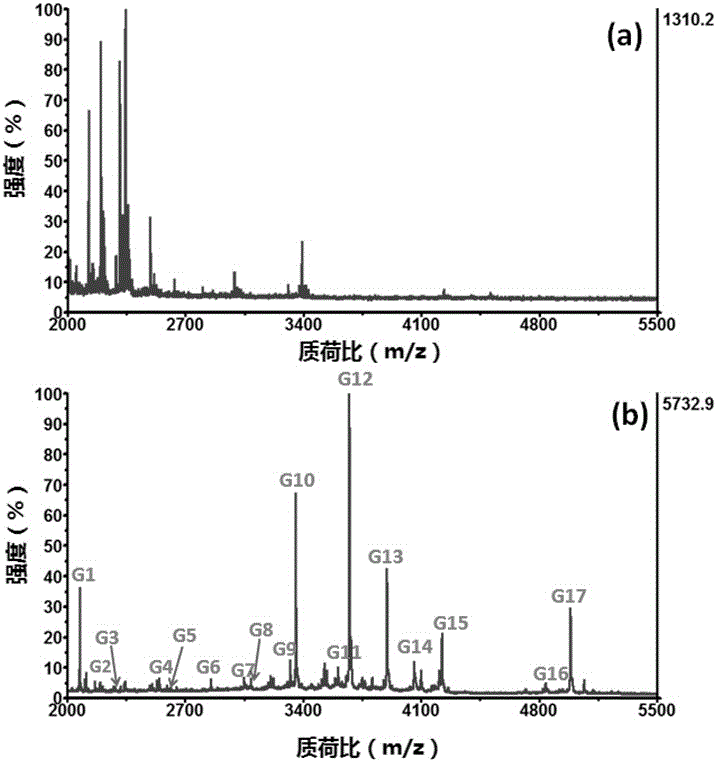
[0045] Example 2: The above metal-organic framework-modified magnetic graphene composite material was applied to the separation, enrichment and MALDI-TOF-MS detection of glycosylated peptides in the standard HRP enzymatic hydrolysis solution.
[0046] (1) Preparation of standard HRP protein enzymatic solution: Accurately weigh 1mg of standard protein HRP and dissolve in 25mM ammonium bicarbonate buffer, boil for 8 minutes, dilute to 1mg / mL with 25mM ammonium bicarbonate buffer, and then Add an appropriate amount of trypsin at a ratio of 1:40, and digest overnight at 37°C for 16 hours;
[0047] (2) Wash 5 mg of the metal-organic framework-modified magnetic graphene composite with 85% acetonitrile / 1.0% trifluoroacetic acid buffer three times, and then disperse in 500 μL of 85% acetonitrile / 1.0% trifluoroacetic acid buffer , ultrasonically disperse until uniform, and prepare a 10mg / mL solution;
[0048] (3) Enrichment of glycosylated peptides: take 50 μL of the solution obtained...
Embodiment 3
[0051] Example 3: The magnetic graphene composite material modified by a metal organic framework obtained in Example 1 is applied to the enrichment and MALDI-TOF-MS of HRP enzymolysis solution and HRP protein and bovine serum albumin (BSA) protein mixed solution detection.
[0052] (1) Mix the HRP enzymatic solution with the HRP and BSA protein solution according to the protein mass ratio of 1:800:800, take 2 μL of the standard mixed solution and add it to 150 μL of 85% acetonitrile / 1.0% trifluoroacetic acid buffer solution, and then add 50 μL of The dispersion of metal-organic framework-modified magnetic graphene composites (concentration: 10 mg / mL) was incubated and swirled at 37°C for 30 minutes; the material was separated by centrifugation and washed three times with 200 μL of 85% acetonitrile / 1.0% trifluoroacetic acid buffer Afterwards, elute with 10 μL 30% acetonitrile / 0.1% trifluoroacetic acid buffer for 15 minutes, and magnetically separate;
[0053] (2) Spotting the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com