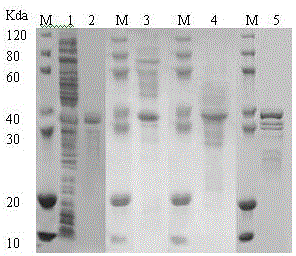
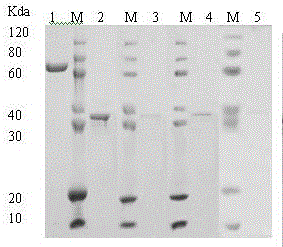
Production method of recombinant glutamine transaminase
A glutamine and production method technology, applied in the field of separation and purification of biological and recombinant proteins, can solve the problems of difficulty in screening high-yield TGase strains, high production costs, and restrictions on industrial production, and achieve stable protein structure and good fragmentation rate , the effect of improving the success rate of renaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 This example illustrates the construction of transglutaminase recombinant Escherichia coli
[0035] 1. Design synthesis with EcoR I Upstream primers with restriction sites and Bam H I The downstream primer of restriction site (the underlined part is the corresponding restriction site),
[0036] Primer1 upstream primer: 5'- GGTCGTCAACAACTACATAC-3';
[0037] Primer2 downstream primer: 5'-GTTCGCCAGTTCCTTCTT-3'.
[0038] 2. According to the MTG gene reported by Fu, R.Y. (GenBank: DQ132977), to Streptomyces mobaraensis Genomic DNA was used as a template, and the target gene fragment was amplified by PCR. The reaction conditions were: 95°C for 5 min; (95°C for 30 s, 60°C for 60 s, 72°C for 45 s, 30 cycles); 72°C for 10 min. Purified and amplified mtg gene (refer to Takara DNA Fragment Purification Kit instructions) and expression plasmid pET30a (purchased from Takara Company) were digested with EcoR I and bamH I respectively, ligated and transformed into ...
Embodiment 2
[0040] Example 2 This example illustrates the fermentation control process of recombinant glutamine transaminase Escherichia coli
[0041] Pick a single clone on the flat plate of the recombinant transglutaminase Escherichia coli BL21(DE3) / mtg obtained in Example 1 and inoculate it in a test tube containing 4 ml of LB culture solution at 37°C and cultivate it at 200 rpm for 16h, then culture a well The grade seed solution was inoculated at 2% inoculum amount in an Erlenmeyer flask containing 200ml LB culture solution and cultivated at 37°C for 16h as the seed solution for fermentation. Kanamycin, peptone 12g / L, yeast extract 24g / L, glycerol 5g / L, K 2 HPO 4 ·3H 2 O 15.2g / l, KH 2 PO 4 2.33g / l) in a 10L fermenter at 37°C, the rotation speed is set to 300rpm, the tank pressure is maintained at 0.03-0.05Mpa, and the initial ventilation volume is 1.5m 3 / h, maintain DO greater than 25% by adjusting the ventilation, cultivate until the OD600 is between 2-3, add a final concentr...
Embodiment 3
[0042] Example 3 This example illustrates the purification of recombinant transglutaminase protein
[0043] Take 26.7g of the collected cells and add 10 times the volume (260ml) of 50mmol / L Tris-HCl (pH 8.0), 0.15mol / L NaCl, 0.5% Triton X-100, 1mM DTT, 0.2mM PMSF, 1mM EDTA and stir well Finally, it was slowly fed into the high-pressure homogenizer, and the pressure was controlled at 800Mpa. After two cycles, the homogenate was centrifuged at 6000rpm at 4°C, and the precipitate was added to 10 times the volume of 50mmol / L Tris-HCl (pH 8.0), 0.15 mol / L NaCl, 0.5% Triton X-100, 1mM DTT, 2mol / L urea, after stirring well, slowly poured into the high-pressure homogenizer, the pressure was controlled at 800Mpa, after two cycles, the homogenate was mixed at 6000rpm, 4℃ Centrifuge, then add 10 times the volume of 50mmol / L Tris-HCl (pH 8.0), 0.15mol / L NaCl, 0.5%Triton X-100, 1mM DTT, 4 mol / L urea to the obtained precipitate, stir well, and flow slowly Add it to a high-pressure homogeni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com