Real-time fluorogenic quantitative PCR detection kit for bovine viral diarrhea-diarrhoea virus and special primer and probe thereof
A bovine viral diarrhea, real-time fluorescence quantitative technology, applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc. The effect of safety and rationality, guaranteed safety, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1, design detects the primer and TaqMan probe of type I and type II bovine viral diarrhea mucosal disease virus with real-time fluorescence quantitative PCR technique
[0056] Since the nucleotide sequences of type I and type II BVDV viruses are relatively different, it is key to find a good site for simultaneous detection of type I and type II BVDV. From NCBI's nucleic acid database GenBank (http: / / www.ncbi.nlm.nih.gov), the 5'UTR region genes of bovine viral diarrhea mucosal disease virus type I and type II (BVDV type I GenBank number: JN704144, Its full gene sequence is shown in SED ID NO: 4 in the sequence listing, BVDV type II GenBank number: FJ431191.1, and its 5' end gene sequence is shown in SED ID NO: 5 in the sequence listing), using DNA Man After the comparison by the software, according to the design principles of primers and TaqMan probes, the 5'UTR region genes of bovine viral diarrhea mucosal disease virus type I and type II were selected (this...
Embodiment 2
[0062] Embodiment 2, carry out real-time fluorescent quantitative PCR detection to type I and type II bovine viral diarrhea mucosal disease virus with primers of the present invention and TaqMan probe
[0063] 1. Extract the genomic RNA of the sample to be tested
[0064] Genomic RNA is extracted from type I and type II bovine viral diarrhea mucosal disease virus cell cultures (for obtaining standard items and obtaining positive controls) and samples to be tested, and the specific method includes the following steps (Axyprep TM Body FluidViral DNA / RNA Miniprep Kit, AXYGEN Company):
[0065] (1) AXYGEN kit preparation: According to the kit instructions, pre-configure isopropanol containing 1% glacial acetic acid and add absolute ethanol of specified concentration to reagent Buffer W1A and Buffer W2.
[0066] (2) Add 200uL of the sample to be tested in a 1.5mL centrifuge tube, and add 200uL of Buffer V-L, vortex and mix well, and let stand for 5min.
[0067] (3) Add 75uL Buffe...
Embodiment 3
[0094] Embodiment 3, specificity experiment
[0095] The AxyGen kit that utilizes DNA / RNA to extract simultaneously according to the method described in Example 2 extracts RNA to type I and type II bovine viral diarrhea mucosal disease virus (BVDV), swine fever virus (CSFV), and to porcine circovirus 2 Type (PCV2) and rhinotracheitis virus (IBRV) to extract DNA, and at the same time, RNA extracted from inactivated type I and type II bovine viral diarrhea virus cell cultures was used as positive control, and enzyme-free water was used as negative control. Under the guidance of the primers and TaqMan probes of the present invention, real-time fluorescent quantitative PCR detection was carried out, and the PCR reaction system and reaction conditions were referred to Example 2 to verify the specificity of the method.
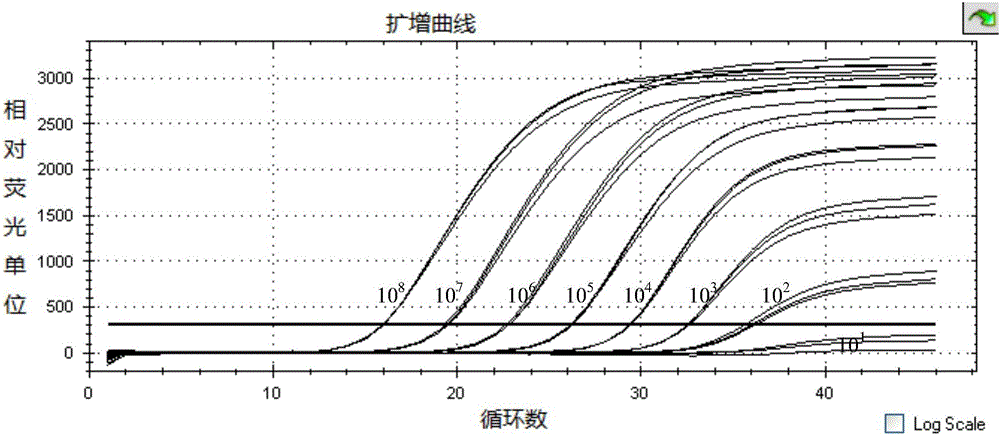
[0096] Test results such as Figure 4 As shown, only BVDV had a specific "S" type amplification curve, and the result was positive, while other samples did not app...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com