Targeted drug delivery system for brain glioma as well as preparation method and use thereof
A glioma and nano-drug delivery system technology is applied to the glioma targeted drug delivery system modified by nucleic acid aptamer AS1411 and the field of preparation thereof, and can solve the problem of low drug loading, neurotoxicity and side effects, and excipients. Problems such as large injection dose to achieve the effect of inhibiting tumor growth and enhancing the inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of polyglutamyl glutamic acid PGG:
[0051] 1) Sodium polyglutamate cPGA-Na (molecular weight 35000, 10g), tert-butyl butyl glutamate hydrochloride H-Glu (otBu) 2 HCl (38.3g, 0.148mol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride EDC HCl (37.0g, 0.193mol), 1-hydroxybenzo Triazole HOBt (10.6 g, 0.074 mol) was dissolved in 500 mL of anhydrous N,N-dimethylformamide DMF, magnetically stirred at 700 rpm at 25°C for 24 h, and protected by argon. The reacted solution was slowly poured into 3 L of deionized water, and a white precipitate was precipitated, filtered, washed three times with 250 mL of deionized water, and freeze-dried for 15 hours to obtain a white solid.
[0052] 2) Transfer the product (60 g) obtained in step 1) to a 1 L round bottom flask, add 480 mL of trifluoroacetic acid TFA, and stir magnetically at 25° C. and 700 rpm for 4 h. TFA was removed by vacuum rotary evaporation at 40°C to obtain a yellow oily substance. After repeatin...
Embodiment 2
[0054] Preparation of polyglutamyl glutamic acid PGG-PTX:
[0055] Add polyglutamine glutamic acid PGG (10g) into 500mL of anhydrous N,N-dimethylformamide DMF, stir magnetically at 25°C and 700rpm for 30min until completely dissolved, then add 1-(3-dimethylaminopropyl base)-3-ethylcarbodiimide hydrochloride EDC·HCl (9.4g, 0.049mol) and 4-lutidine (2.6g), and continued to stir for 15min. Add paclitaxel PTX (5.4g) into the reaction bottle, and stir magnetically at 25°C and 700rpm for 26-28h. After the reaction is complete, slowly pour the reaction solution into 1.5L hydrochloric acid aqueous solution (0.2M) in an ice-bath environment, and a white precipitate precipitates, 5000rpm After centrifugation for 10 min, the resulting white precipitate was dissolved in 1.5 L of sodium bicarbonate (0.5 M) aqueous solution, and then transferred to a dialysis bag (MWCO 10,000) for dialysis for 24 h, during which all deionized water was replaced every 4 hours. When the resistivity of the so...
Embodiment 3
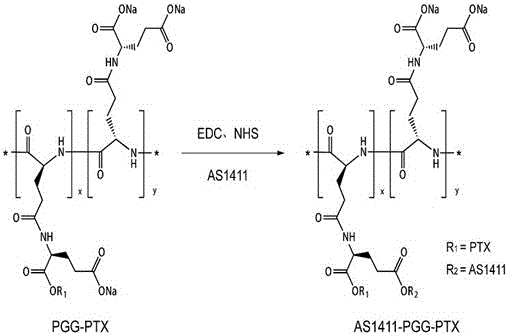
[0058] Preparation of polyglutamine glutamic acid-paclitaxel covalent bond AS1411-PGG-PTX modified by nucleic acid aptamer AS1411:
[0059] 1) Dissolve 8mg (10nmol) of polyglutamine glutamic acid-paclitaxel covalent bond PGG-PTX in 1mL of 2-(N-morpholine)ethanesulfonic acid buffer solution MES buffer (pH 6.0, 0.1M) , where the concentration of PGG-PTX was 10 μM. Then add 200mM 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride EDC·HCl and 200mM N-hydroxysuccinimide NHS in sequence, stir at 25°C and 400rpm After 30 minutes, the carboxyl groups on the surface of PGG-PTX were activated, and the mixed solution changed from transparent and clear to white and turbid.
[0060] 2) Transfer the solution obtained in step 1) to a 4mL ultrafiltration tube (molecular weight cut-off: 30000), wash with DNA / RNase-free distilled water DNase / RNase free water, remove EDC / NHS, and use 4mL of DNase / RNase free water / time, the centrifugal force is 2500g, 5min / time, repeat this step 4 tim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com