Magnetic composite nanoparticles and preparation method and application thereof
A magnetic composite nanometer and nanoparticle technology is applied in the field of avidin-modified nucleic acid functionalized magnetic composite nanoparticles and their preparation, which can solve the problems of lack of cancer cell release regulation, difficult nuclease activity analysis and in situ imaging, etc. , to achieve the effect of uniform particle size and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
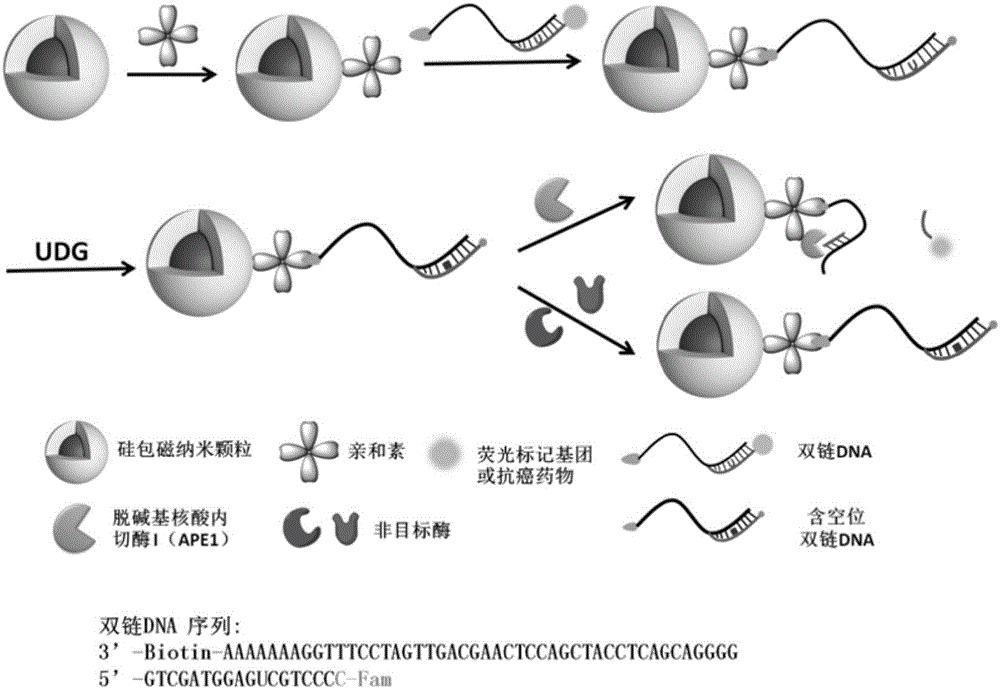
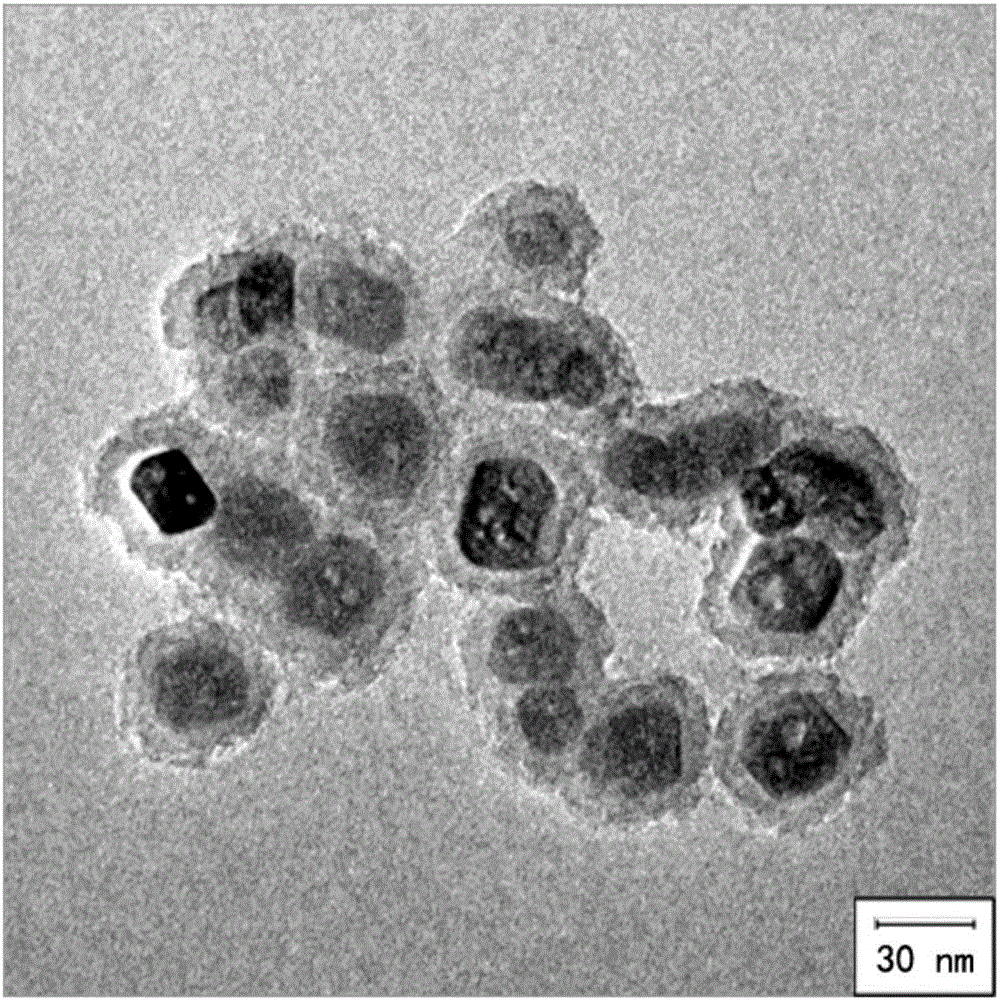
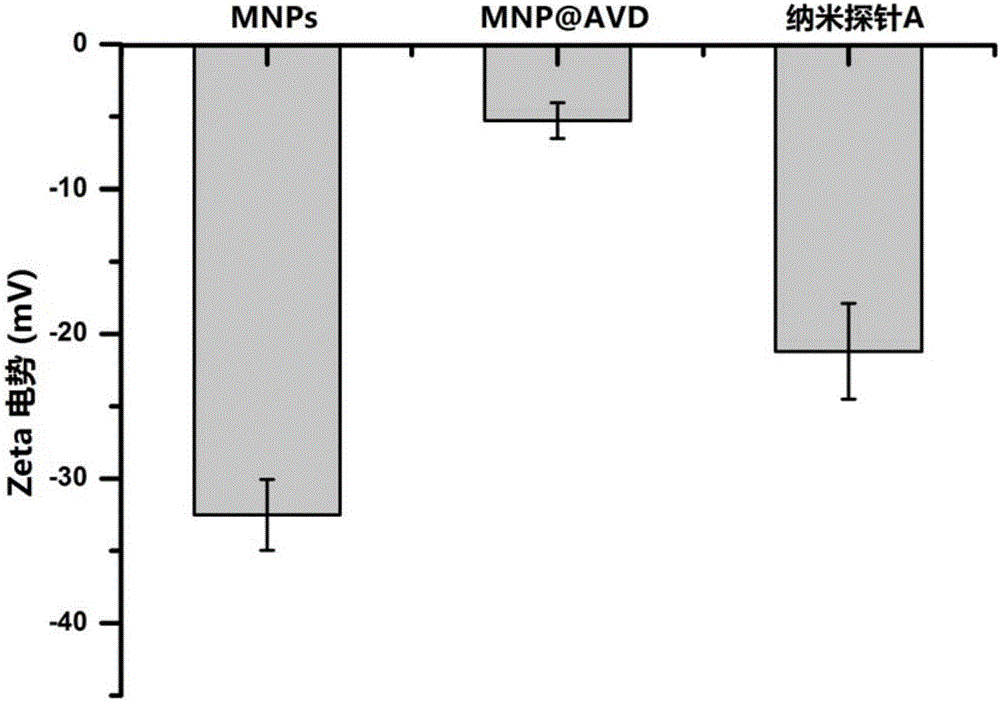
[0048] Embodiment 1, preparation magnetic composite nanoparticle Fe 3 o 4 @SiO 2 @AVD-AP-DNA (Nanoprobe A)
[0049] The process of preparing magnetic composite nanoparticles in the present invention is as follows: figure 1 As shown, it specifically includes the following steps:
[0050] 1.0mg Fe 3 o 4 @SiO 2 At room temperature, under 40KHz (this ultrasonic frequency is used in the present invention, it can be adjusted to a different ultrasonic time as required) ultrasonic for 20 minutes, dispersed in 2.0mL pure water to form a 0.5mg / mL solution, add 4.0mg EDC and 10.0 mg NHS to activate the carboxyl group, shake the reaction at room temperature for 30 minutes. Add 0.1 mL of avidin with a concentration of 2.0 mg / mL, and shake and react at room temperature for 8 hours. Magnetic separation, removal of unreacted substances, washing with deionized water three times, re-sonication dispersion in 2.0 mL of pure water. Add biotinylated double-stranded DNA containing U base at...
Embodiment 2
[0052] Embodiment 2, the APE1 activity in the solution is directly detected
[0053]In this embodiment, the DNA probe sequence of design is as follows:
[0054] 5'-GGGGACGACTCCATCGACCTCAAGCAGTTGATCCTTTGGAAAAAAA-Biotin-3'
[0055] 5'-GTCGATGGAGXCGTCCCC-FAM-3'
[0056] Among them, X represents the abasic site, Biotin represents biotin labeling, and FAM represents carboxycarboxyfluorescein labeling.
[0057] The experimental steps are as follows:
[0058] 1. In a 200 μL PCR tube, add 10 μL of the 0.5 mg / mL magnetic composite nanoparticle solution prepared in Example 1, 5 μL of 10× Buffer1.1 buffer, add deionized water to make the total volume 48 μL, and then add 2 μL Different concentrations of APE1 solutions, so that the final concentrations of APE1 were 0, 0.01, 0.05, 0.1, 0.2, 0.5, 1U / mL, and the response of the composite nanoparticles was detected.
[0059] Stratagene Mx3000P fluorescent PCR instrument was used to collect fluorescence every 5s at 37°C, the excitation wave...
Embodiment 3
[0064] Example 3. Composite nanoparticles are quickly introduced into cells under the action of a magnetic field and release fluorescent signals
[0065] The prepared magnetic composite nanoparticles have good stability and biocompatibility, and can be quickly imported into Hela cells through endocytosis under the action of a magnetic field to image APE1.
[0066] The experimental steps are as follows:
[0067] 1. Cultivate cells in DMEM medium containing 1% penicillin-streptomycin double antibody and 10% inactivated fetal bovine serum, the incubator conditions are 37°C and 5% CO 2 / 95% air. When the cells grow to cover 70%-80% of the culture flask, add 0.25% trypsin and treat at 37°C for 4 minutes, then transfer the cells to a 96-well flat-bottom glass plate, each well contains 0.1mL cell culture medium, about 10 4 cells. The cells were cultured for 24 h to allow them to adhere to the wall before the experiment.
[0068] 2. The cells were washed with DPBS buffer, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com