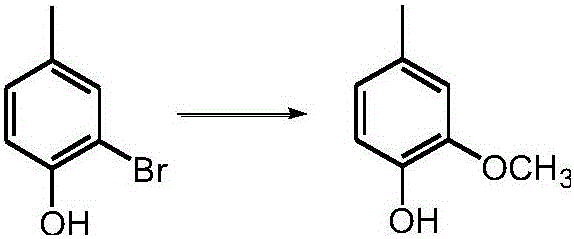
Method of synthesizing 2-alkoxyl-4-methylphenol with 2-bromine-4-methylphenol
A technology of methyl phenol and alkoxy group, applied in the field of fine chemical industry, can solve the problems of low overall yield, incomplete reaction, difficult industrialized production, etc., and achieve the effect of high atom utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
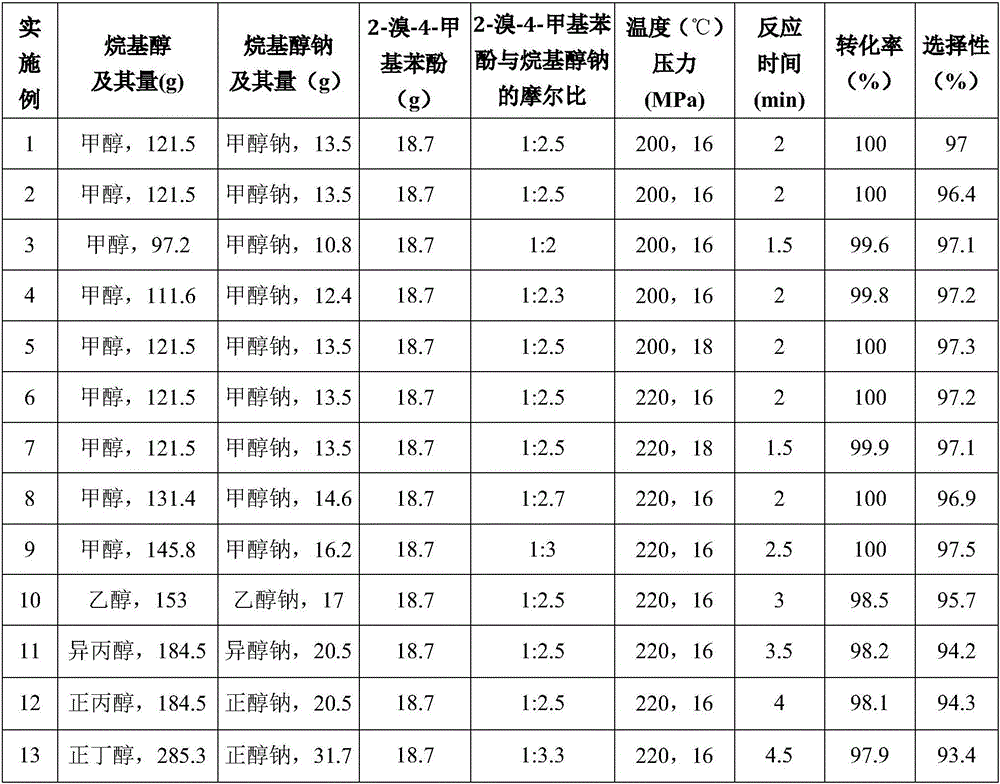
[0043] Under normal temperature and pressure operating conditions, first add a solution of 13.5g sodium methoxide and 121.5g methanol into the high-pressure reactor, pressurize to 16MPa, and heat to 200°C to reach the near-critical state of methanol, and then pass through the high-pressure liquid phase pump 18.7 g of 2-bromo-4-methylphenol liquid at a temperature of 80° C. was quickly pumped into the autoclave to carry out alkoxylation reaction for 2 minutes, and then the resulting reaction liquid was decompressed and cooled to normal temperature and pressure. Remove and recover methanol from the resulting reaction solution by distillation under reduced pressure, add sulfuric acid solution to the remaining reaction solution to carry out acidification treatment to make the pH value of the reaction solution 1, then wash, stand and separate, the upper layer solution is light red 13.38 g of clear liquid product.
[0044] Adopt gas phase mass spectrometry to confirm that the obtain...
Embodiment 2
[0046] First add the solution of 13.5g of sodium methoxide and 54g of methanol into the autoclave to make the methanol reach a near-critical state, then pump the solution of 67.5g of methanol and 18.7g of 2-bromo-4-methylphenol into the In the autoclave, 13.3 g of a light red transparent liquid product was prepared in the same manner as in Example 1.
[0047] It can be confirmed that the obtained product is 2-methoxy-4-methylphenol by gas phase mass spectrometry, and the conversion rate of raw material 2-bromo-4-methylphenol is measured by the same gas chromatography internal standard method as in Example 1. %, the selectivity for the product 2-methoxy-4-methylphenol is 96.4%. The measurement results are shown in Table 1.
Embodiment 3-9
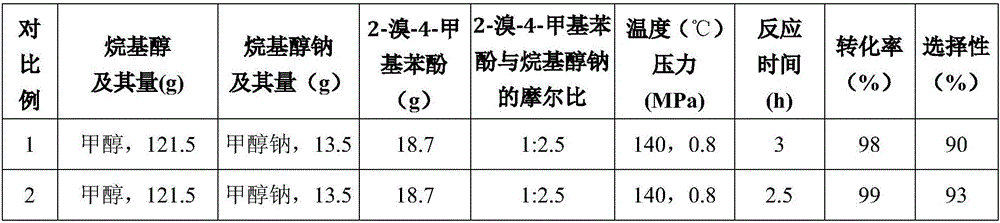
[0049] Except changing conditions such as methanol consumption, sodium methylate consumption, reaction temperature, reaction pressure, reaction time, the molar ratio of 2-bromo-4-methylphenol and sodium methylate as shown in Table 1, with the method identical with embodiment 1 Light red transparent liquid products were obtained, respectively 13.35g, 13.39g, 13.43g, 13.41g, 13.39g, 13.37g, 13.46g.
[0050] Confirm that the product obtained in Examples 3-9 is 2-methoxyl-4-methylphenol by gas phase mass spectrometry, and measure the content of raw material 2-bromo-4-methylphenol by using the same gas chromatography internal standard method as in Example 1. Conversion and selectivity to the product 2-methoxy-4-methylphenol. The measurement results are shown in Table 1.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap