Preparation method 1,1,1,4,4,4-hexafluoro-2-butene
A technology of hexachlorobutadiene and butene, which is applied in the field of preparation of fluorine-containing olefins, can solve problems such as high pressure, difficulty in recycling amide solvents, and many wastes, and achieve the effects of large operating flexibility, easy industrialization, and reduction of wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
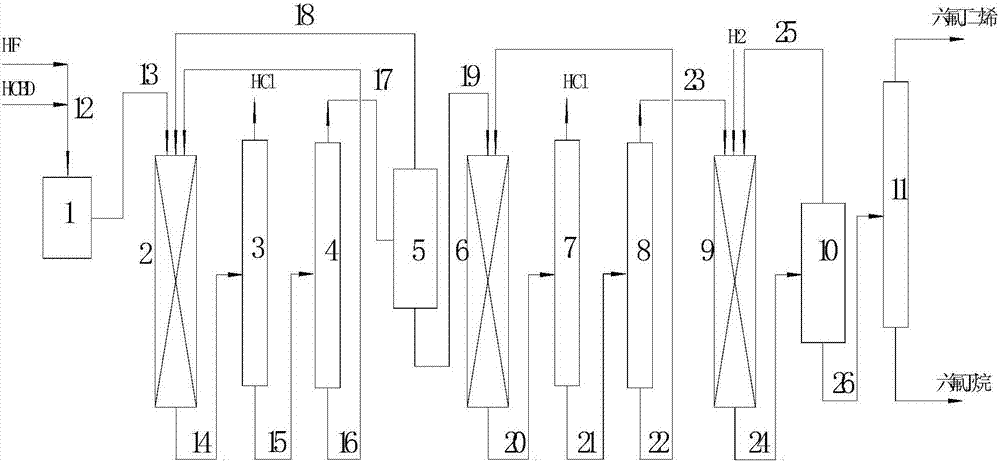
[0055] 120ml Cr 2 o 3 / Zn / Mg / Ga catalyst (the loading of Zn is 3wt%, the loading of Mg is 1wt%, and the loading of Ga is 0.1wt%) is loaded into the first reactor, and 200ml Al 2 o 3 / ZnCl 2 Catalyst (ZnCl 2 The load is 3wt%) into the second reactor, 200mlPd / BaSO 4 Catalyst (0.1 wt% Pd loading) was charged into the third reactor. Heat the first reactor to 150°C for drying, continue to heat up to 350°C for 4 hours under the protection of nitrogen flow rate of 2.5L / min, then cool down to 300°C, and feed HF at a flow rate of 50g / h, the heat release is obvious, and the hot spot goes away After completion, the temperature was raised to 350°C and HF was fed into the flow rate of 100g / h to continue fluorination for 40 hours; the second reactor was heated to 350°C, the nitrogen flow rate was 2.5L / min, and solidified for 4 hours; the third reactor was passed into H 2 , the flow rate is 0.4L / min, the temperature is raised to 250°C, and it is stable for 2 hours. Catalyst treatment ...
Embodiment 2
[0060] 120ml Cr 2 o 3 / Zn / Mg / Ga catalyst (the loading of Zn is 5wt%, the loading of Mg is 3wt%, and the loading of Ga is 0.5wt%) is loaded into the first reactor, and 200ml Al 2 o 3 / ZnCl 2 Catalyst (ZnCl 2 The load is 10wt%) into the second reactor, 200mlPd / BaSO 4 Catalyst (0.2 wt% Pd loading) was charged to the third reactor. The catalyst treatment methods of the three reactors are the same as in Example 1.
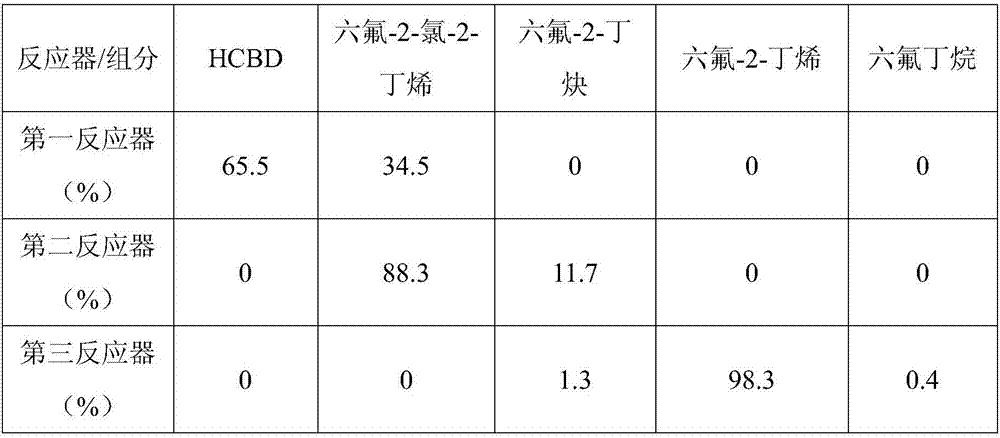
[0061] Then start the feeding reaction, the molar ratio of HF and HCBD is 15:1, the bed temperature of the first reactor is 230°C, and the space velocity is 500h -1 , the mixture at the outlet of the first reactor is analyzed by gas chromatography for organic composition; the 1,1,1,4,4,4-hexafluoro-2-chloro-2-butene obtained by the hydrogen fluoride separator enters the second reactor and is controlled Bed temperature 300°C, space velocity 200h -1 , the mixture at the outlet of the second reactor is analyzed by gas chromatography for organic composition; the sec...
Embodiment 3
[0065] 120ml Cr 2 o 3 / Zn / Mg / Ga catalyst (the loading of Zn is 5wt%, the loading of Mg is 3wt%, and the loading of Ga is 1wt%) is loaded into the first reactor, and 200ml Al 2 o 3 / ZnCl 2 Catalyst (ZnCl 2 The load is 10wt%) into the second reactor, 200mlPd / BaSO 4 Catalyst (0.2 wt% Pd loading) was charged to the third reactor. The catalyst treatment methods of the three reactors are the same as in Example 1.
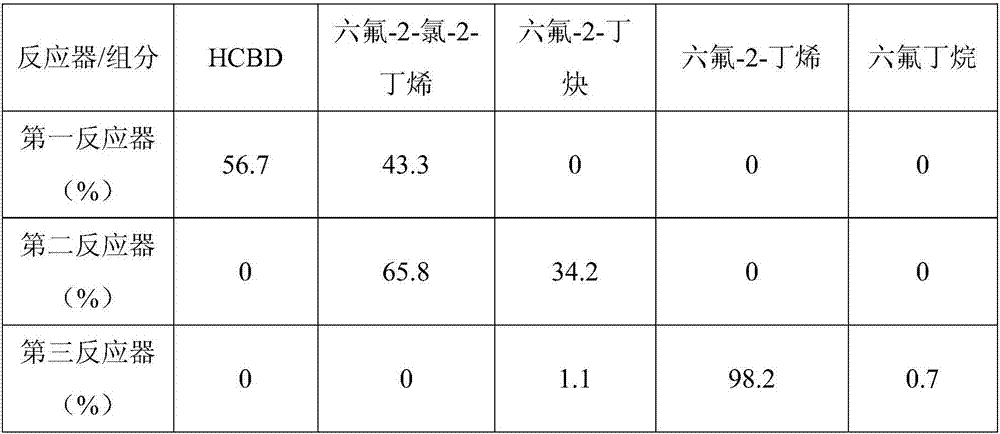
[0066] Then start the feeding reaction, the molar ratio of HF and HCBD is 15:1, the bed temperature of the first reactor is 260°C, and the space velocity is 500h -1 , the mixture at the outlet of the first reactor is analyzed by gas chromatography for organic composition; the 1,1,1,4,4,4-hexafluoro-2-chloro-2-butene obtained by the hydrogen fluoride separator enters the second reactor and is controlled Bed temperature 350°C, space velocity 400h -1 , the mixture at the outlet of the second reactor is analyzed by gas chromatography for organic composition; the secon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com