Naphthyl two-photon fluorescent probe as well as preparation method and application thereof
A two-photon fluorescence and probe technology, applied in the field of fluorescent probes, can solve the problems of limited deep tissue imaging applications, lack of mitochondrial targeting, large photodamage and photobleaching, etc., achieves good cell membrane permeability, and is conducive to Commercial production, good selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthetic route of naphthyl two-photon fluorescent probe:
[0032]
[0033] Synthesis steps and characterization:
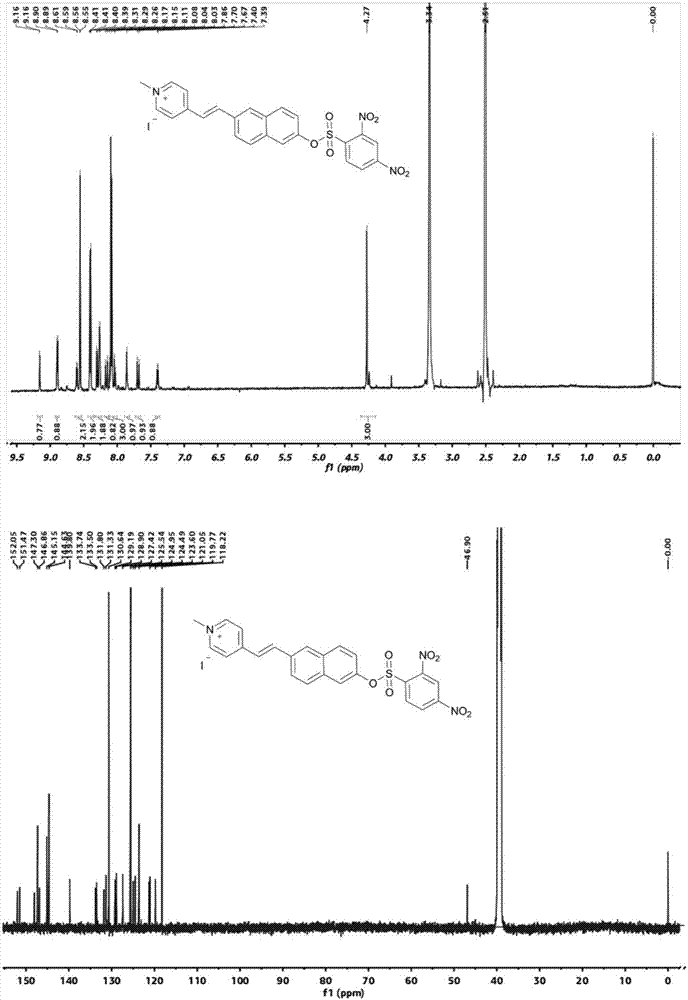
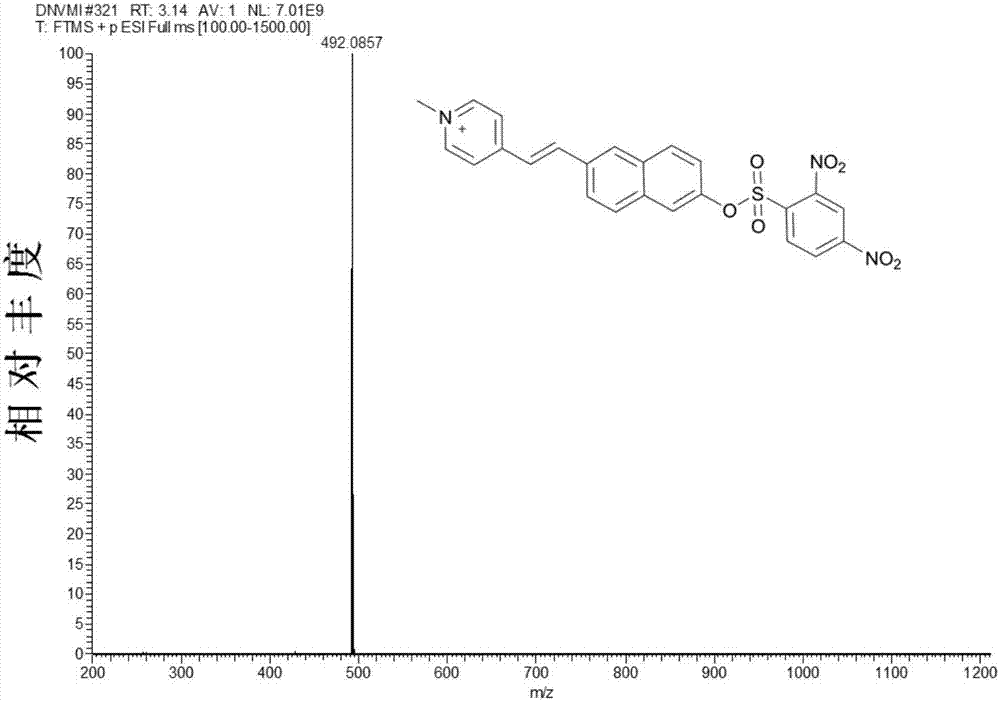
[0034] in N 2 Under protection, 1g (2.56mmol) 1-methyl-4-[2-(6-hydroxy-2-naphthalene)-vinyl]-pyridine iodide salt and 0.14g (2.56mmol) sodium methoxide were added to 50mL anhydrous In methanol, the reaction was heated at 60°C for 4 hours, the color of the solution gradually changed from yellow to blue, and the blue solid was obtained by cooling and filtration, which was directly carried out to the next reaction without further treatment. Add 1g (2.43mmol) of blue solid, 0.78g (2.92mmol) of 2,4-dinitrobenzenesulfonic acid chloride and 0.26g (2.43mmol) of triethylamine into 100ml of anhydrous acetonitrile, and heat at 60°C for 2 Hours, the crude product was obtained by distillation under reduced pressure. The crude product was separated by column chromatography (dichloromethane as the mobile phase) to obtain 1.05 g of a khaki solid with a yield of ...
Embodiment 2
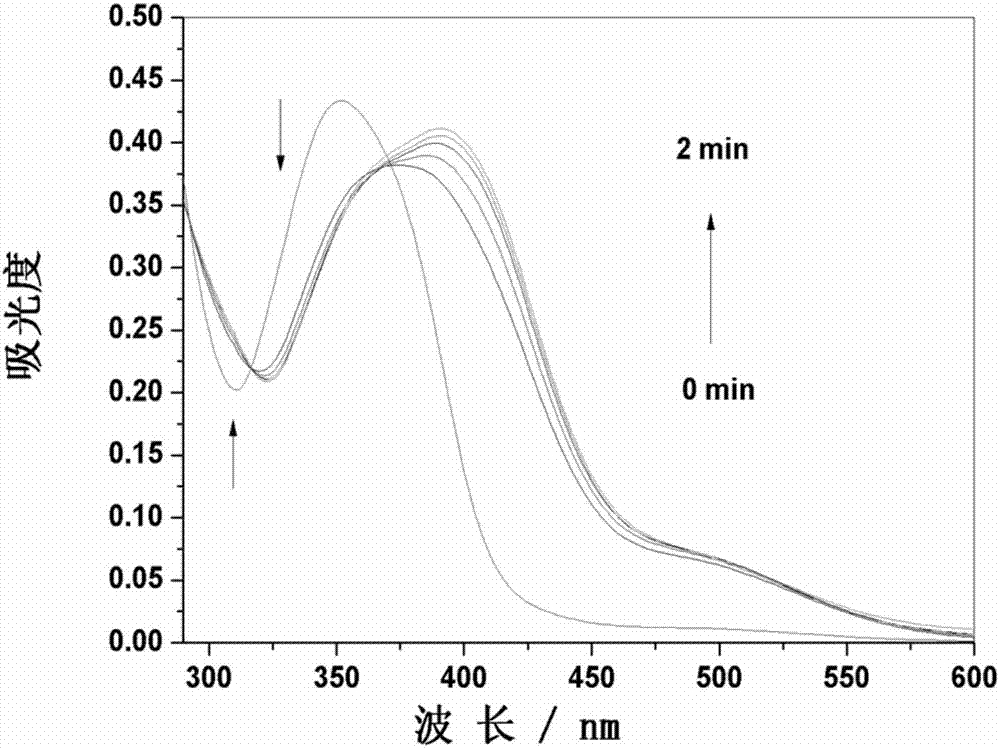
[0036] The probe in Example 1 was used to prepare a stock solution with a concentration of 1 mM in DMSO. Dissolve Cys in secondary water to make a 0.1M solution for later use. In the experiment, the probe was diluted to a final concentration of 10 μM with DMSO / PBS buffer (pH 7.4) system (v / v=1 / 1), and the ultraviolet-visible absorption spectrum of the reaction between the probe and Cys (100 μM) was recorded over time ( image 3 ). With the increase of the reaction time between the probe and cysteine, the maximum absorption peak at 352nm gradually decreases and red shifts to 392nm, an isosbestic point appears at 370nm, and the time for the maximum absorption peak at 392nm is 2min. At the same time, the color of the solution changed from colorless to yellow ( Figure 4 ).
Embodiment 3
[0038] The probe was diluted to 10 μM with DMSO / PBS buffer (pH 7.4) system (v / v=1 / 1), and the fluorescence spectrum spectrum of the reaction between the probe and Cys (100 μM) was recorded over time, and the excitation wavelength was fixed at 370 nm. Excitation and emission slit broadband are both 2.0nm. Such as Figure 5 As shown, before Cys was not added, the maximum fluorescence emission of the probe was located at 485nm. As the reaction time between the probe and cysteine increased, the fluorescence emission at 485nm gradually decreased, and a new fluorescence emission appeared at 583nm and Significantly enhanced with a clear isoelectric point at 502nm. And the reaction reaches the maximum value at 2min, which is faster than most biothiol probes reported in the literature. Under the irradiation of ultraviolet light, the color of the solution changed from light blue to green ( Figure 6 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com