Preparation method of electrochemiluminescent material based on zinc porphyrin
A technology of electrochemistry and luminescent materials, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of poor water solubility, poor biocompatibility, and unsuitable biological applications, and achieve high water solubility and biocompatibility The effect of high stability and stable ECL signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
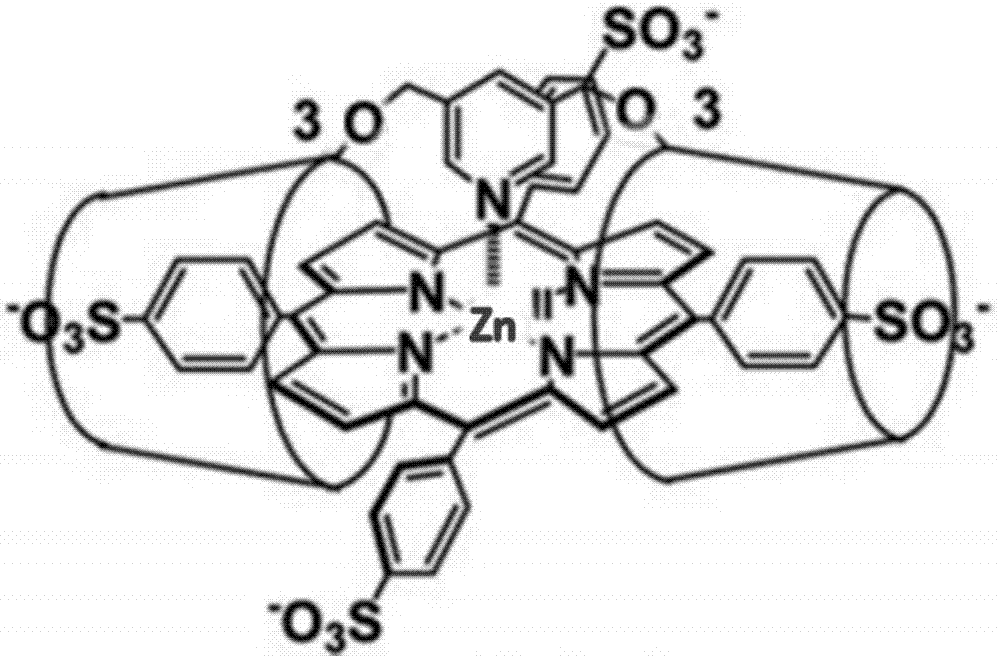
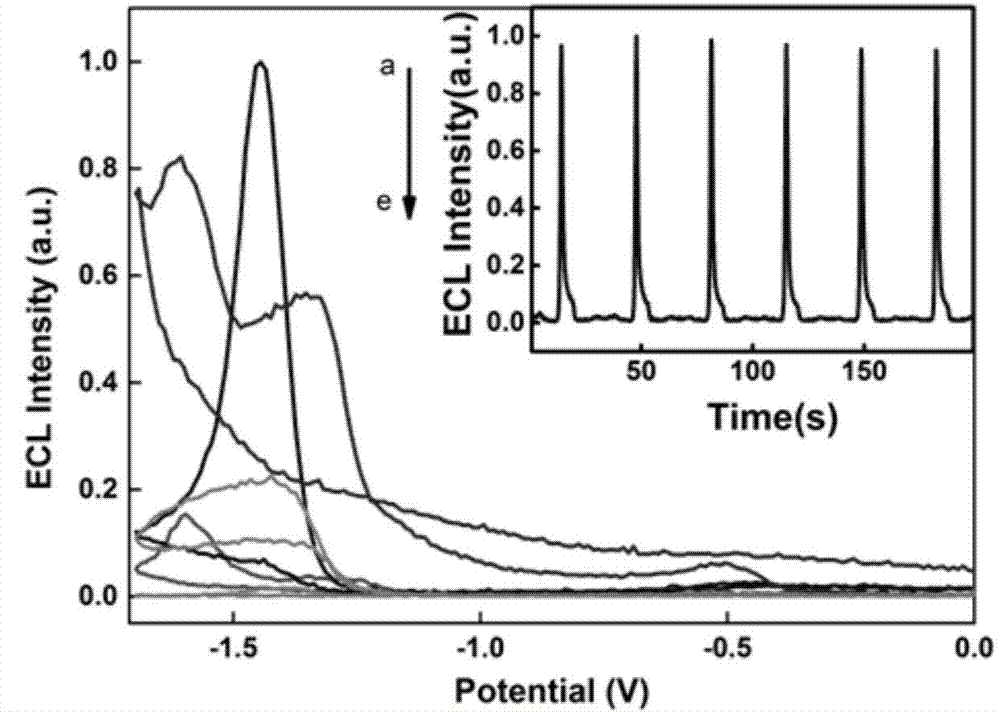
[0024] ECL signal detection of zinc porphyrins.
[0025] 1. The meta-tetraphenylsulfonic acid base zinc porphyrin (a), 2,4-diethylene glycol hypo zinc porphyrin (b), hypo zinc porphyrin (c) and meta-tetrakis (N- Methyl-4-pyridyl) zinc porphyrin (d) and meta-tetrapyridine zinc porphyrin (e) were uniformly prepared into a 0.5 mM aqueous solution, and ultrasonicated at room temperature for 30 min.
[0026] 2. Polish the glassy carbon electrode with a diameter of 5mm on the chamois leather of 0.3μm and 0.05μm alumina respectively for 3 minutes, rinse the alumina adsorbed on the surface of the electrode with ultrapure water each time, and then wash it with acetone, ethanol, deionized Ultrasonic in water, dry with nitrogen gas for later use.
[0027] 3. Add 15 μL of different porphyrin solutions dropwise to the surface of the treated glassy carbon electrode, and dry it in a thermostat at 37° C. for 1 hour to obtain a working electrode.
[0028] 4. With pH 7.4 HEPES solution as the...
Embodiment 2
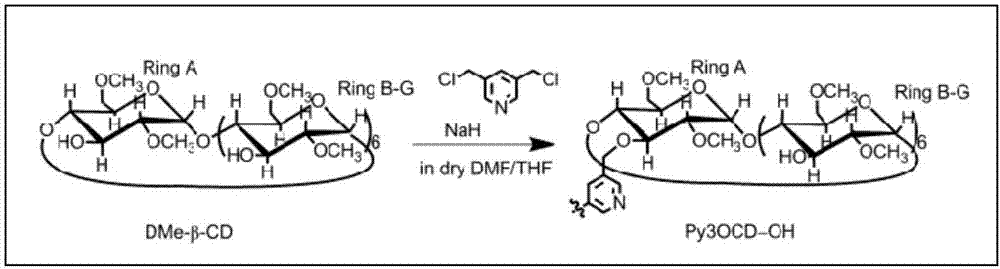
[0031] The synthesis of seven-(2,6-methoxy)-β-cyclodextrin dimer, the synthetic route is as follows image 3 As shown, the specific steps are:
[0032] 1. Weigh 5g of hepta-(2,6-methoxy)β-cyclodextrin (DMe-β-CD) and dissolve it in 80mL of anhydrous tetrahydrofuran to form a white translucent solution, continue stirring, then slowly add 1g containing 60 % sodium hydride in mineral oil (the mass ratio of DMe-β-CD is 5:1), and the mixture was refluxed for 1 h.
[0033] 2. Weigh 0.2g of 3,5-dichloromethylpyridine (the molar ratio of DMe-β-CD is 1:2) and dissolve it in 20mL of dry N,N-dimethylformamide, and use a constant pressure funnel Add it dropwise to the above reaction system over 1 hour, continue to stir the reaction, and reflux overnight.
[0034] 3. After the reaction was completed, methanol was slowly added under ice-bath conditions until the mixture became transparent, and distillation under reduced pressure was carried out. The residue obtained was dissolved in 40 mL ...
Embodiment 3
[0039] The synthesis of seven-(2,6-methoxy)-β-cyclodextrin dimer, the synthetic route is as follows image 3 As shown, the specific steps are:
[0040] 1. Weigh 5 g of seven-(2,6-methoxy) β-cyclodextrin (DMe-β-CD) and dissolve it in 80 mL of anhydrous tetrahydrofuran to form a white translucent solution, continue stirring, and then slowly add 0.5 g containing 60% sodium hydride in mineral oil (3:1 with DMe-β-CD), the mixture was refluxed for 1h.
[0041] 2. Weigh 0.09g of 3,5-dichloromethylpyridine (the ratio of DMe-β-CD is 1:5) and dissolve it in 20mL of dry N,N-dimethylformamide (DMF), and use constant The pressure funnel was added dropwise to the above reaction system over 1 hour, and the stirring reaction was continued, and the mixture was refluxed overnight.
[0042] 3. After the above reaction was completed, methanol was slowly added under ice-bath conditions until the mixture became transparent, and distillation under reduced pressure was carried out. The resulting re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com