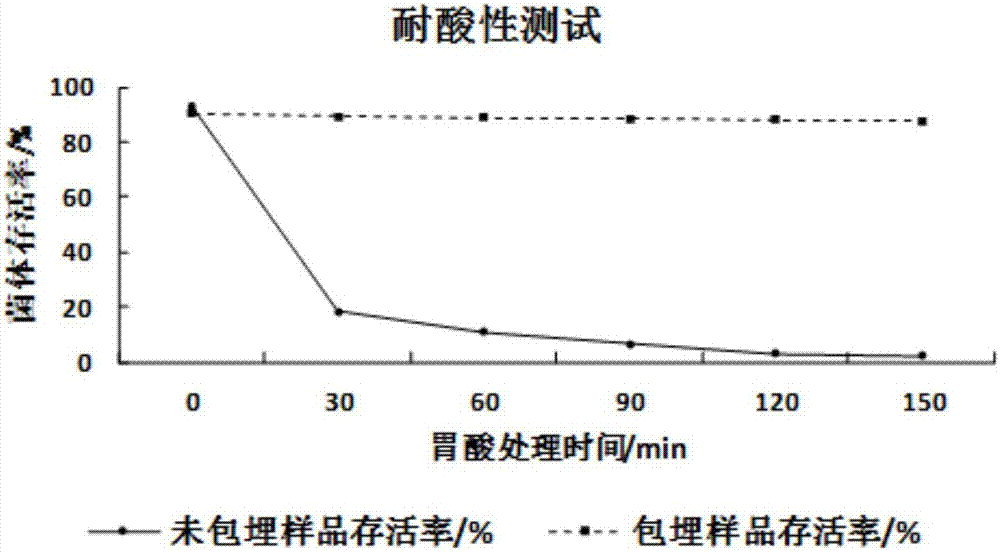
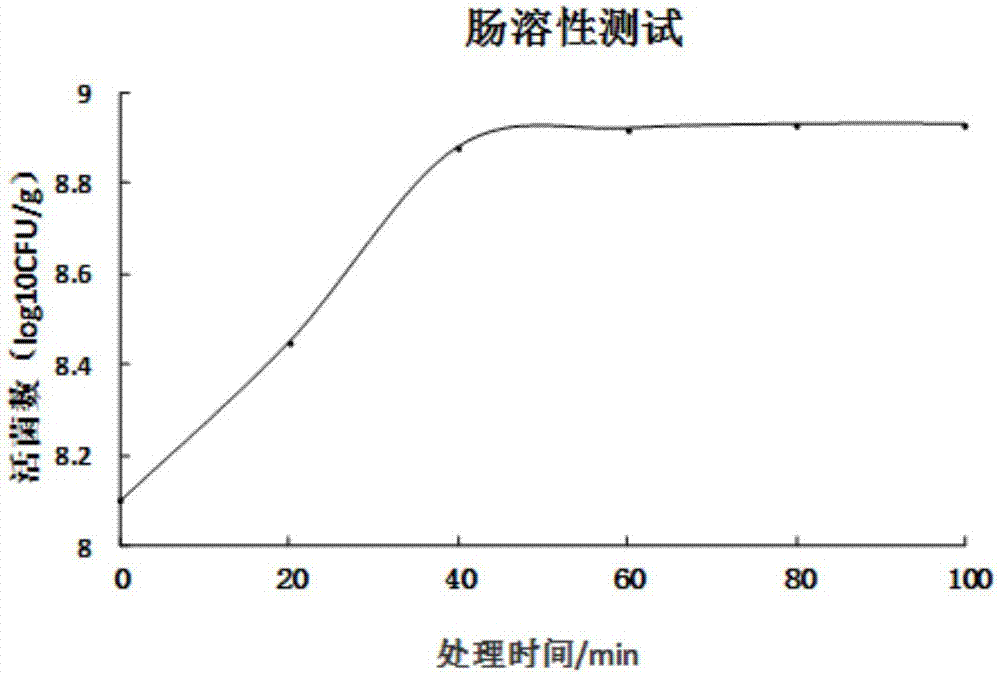
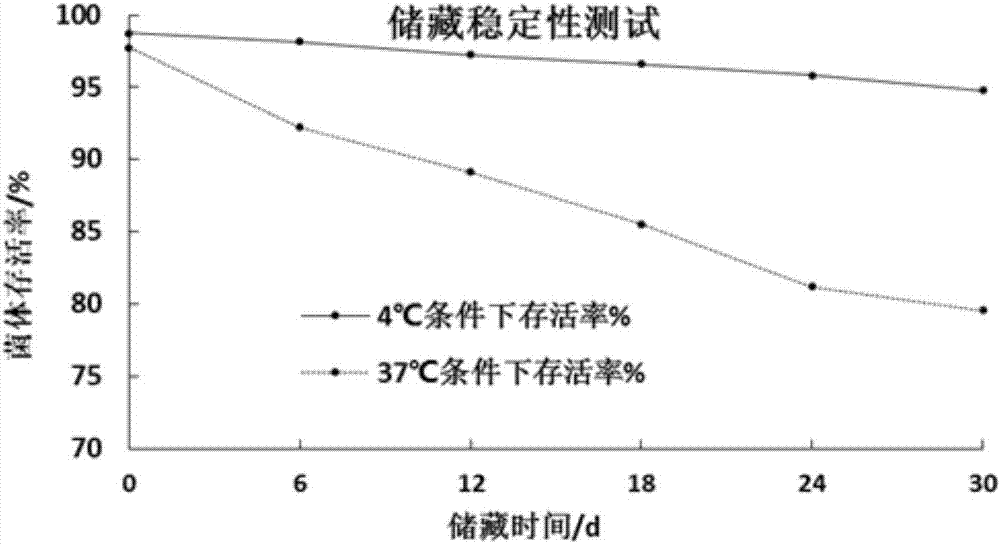
Preparation method of probiotics microcapsule
A technology of microcapsules and probiotics, applied in the field of microorganisms, can solve the problems that probiotic strains cannot be quickly colonized and released, cannot protect the cells, and the microcapsules have poor acid resistance, so as to improve physical stability, rapidly multiply probiotics, reduce effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 bifidobacterium lactis microcapsules
[0035] A preparation process of Bifidobacterium lactis microcapsules, the strains are purchased from the China Industrial Microbiology Strain Preservation Management Center, including the following specific steps:
[0036] (1) Compound freeze-drying protective agent preparation: 0.2% glycerin, 0.5% glycine, 2% lactose, 0.5% sorbitol, 2% bovine serum albumin, constant volume with distilled water, sterilized at 115°C for 20min before use, Use after cooling.
[0037] (2) Preparation of embedding wall material: premix methylcellulose (relative molecular mass: 40kDa) with galacto-oligosaccharide according to the mass ratio of 1:2, then dissolve it in sterile water, and mix it evenly. The Abbe refractometer measures the refractive index, and the refractive index is finally controlled at 1.35. Stir for 15 min with a magnetic stirrer. Put the mixed solution into a radiation-resistant PE plastic bag for se...
Embodiment 2
[0043] The preparation of embodiment 2 Lactobacillus acidophilus microcapsules
[0044] A preparation process of Lactobacillus acidophilus microcapsules, the strains are purchased from the China Industrial Microbiology Strain Preservation Management Center, including the following specific steps:
[0045] (1) Compound freeze-drying protective agent preparation: 0.15% glycerin, 0.2% glycine, 1% lactose, 1% sorbitol, 1.5% bovine serum albumin, constant volume with distilled water, sterilized at 121°C for 15 minutes before use, Use after cooling.
[0046] (2) Preparation of embedding wall material: premix methylcellulose (relative molecular mass: 50kDa) with galacto-oligosaccharide according to the mass ratio of 1:8, then dissolve it in sterile water and mix it evenly. The Abbe refractometer measures the refractive index, and the refractive index is finally controlled at about 1.40. Finally, a magnetic stirrer was used to stir for 20 min. Put the mixed solution into a radiatio...
Embodiment 3
[0051] The preparation of embodiment 3 Bifidobacterium longum microcapsules
[0052] A preparation process of Bifidobacterium longum microcapsules, the bacterial strains are purchased from the China Industrial Microorganism Strain Preservation Management Center, including the following specific steps:
[0053] (1) Compound freeze-drying protective agent preparation: 0.25% glycerin, 0.3% glycine, 1% lactose, 1.5% sorbitol, 2% bovine serum albumin, constant volume with distilled water, sterilized at 118°C for 18 minutes before use, Use after cooling.
[0054] (2) Preparation of embedding wall material: premix methylcellulose (relative molecular mass: 60kDa) with galacto-oligosaccharide according to the mass ratio of 1:6, then dissolve it in sterile water, and mix it evenly. The Abbe refractometer measures the refractive index, and the refractive index is finally controlled at about 1.44. Finally, a magnetic stirrer was used to stir for 20 min. Put the mixed solution into a ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com