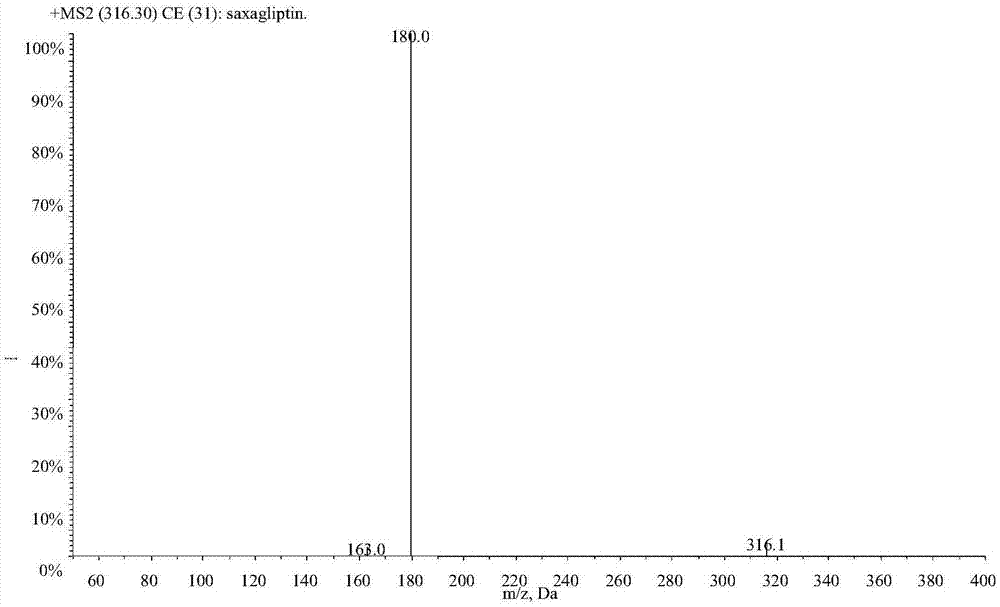
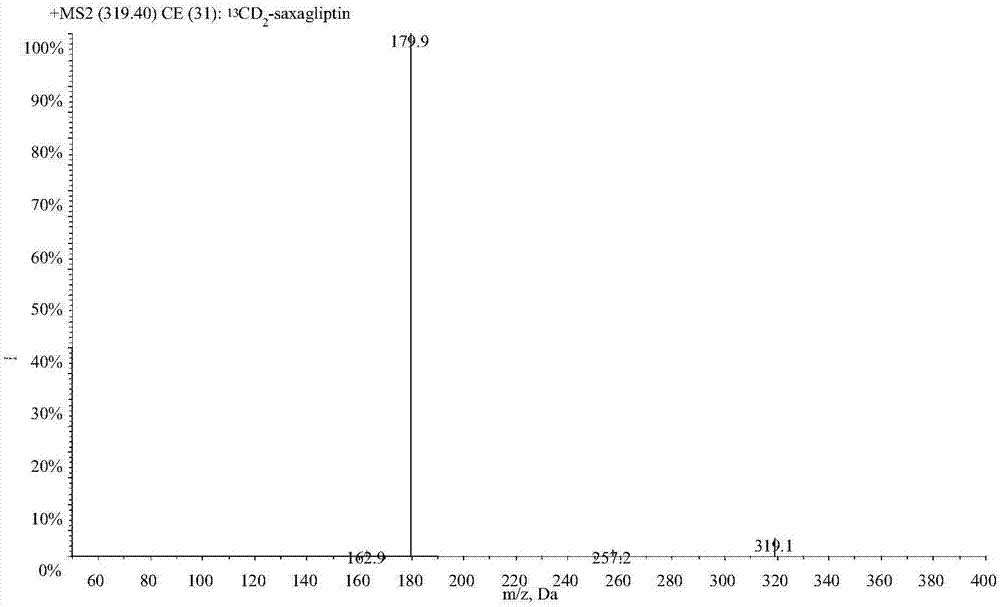
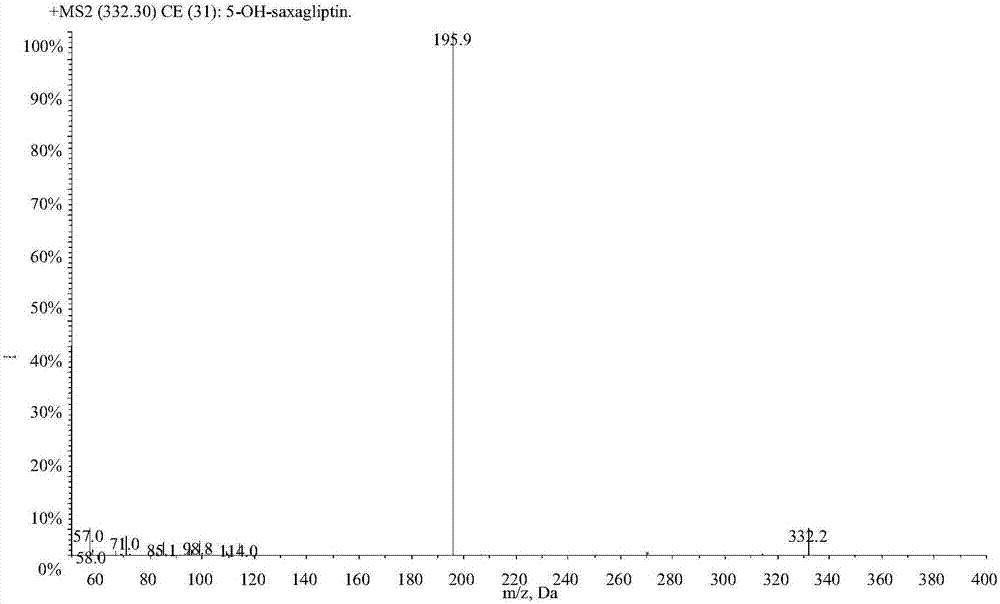
LC-MS/MS high-throughput detection method of saxagliptin and 5-hydroxysaxagliptin in human blood plasma
A technology of hydroxysaxagliptin and detection method, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of increasing blood concentration, shortening running time, and low sensitivity, so as to improve detection accuracy and chromatographic running time Short, the effect of solving the residual problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Establishment of LC-MS / MS determination method for saxagliptin and its metabolite 5-hydroxy saxagliptin in human plasma
[0030] 1. Solution and sample preparation
[0031] 1.1 Standard series samples: Accurately weigh the appropriate amount of each reference substance, dissolve saxagliptin in acetonitrile and constant volume, and dissolve 5-hydroxy saxagliptin in acetonitrile:water (50:50, v / v) and constant volume, and prepare Prepare a stock solution with a concentration of about 1.00 mg / mL, accurately absorb the appropriate amount of each stock solution, and dilute step by step with acetonitrile: water (3:1, v / v) to obtain a mixed standard series of working solutions, and dilute the working solution with human blank plasma , the concentration ranges of saxagliptin and 5-hydroxy saxagliptin in the mixed standard series samples were 0.200-50.0 and 0.298-74.4ng / mL respectively, which were used to draw the standard curve;
[0032] 1.2 Quality control samples: ...
Embodiment 2
[0067] Embodiment two Determination of saxagliptin and its metabolite 5-hydroxyl saxagliptin in human plasma
[0068] Apply the established LC-MS / MS assay method for the content of saxagliptin and its metabolite 5-hydroxy saxagliptin in human plasma to determine the concentration of saxagliptin and 5-hydroxy saxagliptin in plasma for evaluation Bioequivalence study of saxagliptin tablets.
[0069] 48 healthy subjects were enrolled, half male and half male, among them, 24 were administered on an empty stomach, and 24 were administered after meals. The drug group took the test or reference preparations on an empty stomach in a standing position 30 minutes after the start of meal timing, before administration and after administration 0.25h, 0.5h, 0.75h, 1.0h, 1.5h, 2.0h, 3.0h, Collect about 4mL of venous blood at 4.0h, 6.0h, 8.0h, 12.0h, 16.0h, 24.0h, 36.0h, and 48.0h. After the blood sample is collected, it is placed in a test tube that has been labeled in advance, and the plas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com