Narrow-band-gap n-type polymer acceptor and preparation method and application thereof
A polymer and compound technology, applied in the polymer field, can solve the problems of weak light response in the long wavelength direction and low short-circuit current in all-polymer battery devices, achieve high molar absorption coefficient and thermal stability, high filling factor, The effect of wide spectral response range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
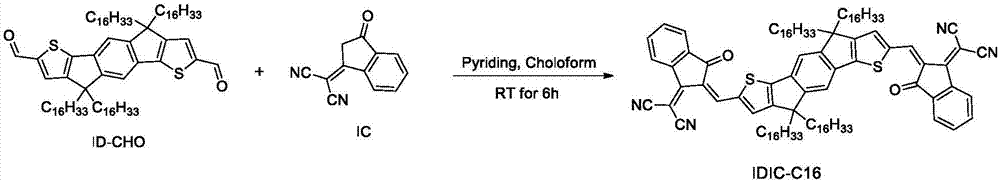
[0072] Embodiment 1, the synthesis of formula IDIC-C16
[0073] Its reaction equation is as figure 1 shown. Concrete reaction steps and reaction conditions are as follows:
[0074]
[0075] Preparation of ID-CHO:
[0076] The preparation method of ID-CHO can be prepared by referring to the literature (Zhang Zhiguo, Li Yongfang, J.Am.Chem.Soc. 2016, 138, 15011), just replace m-IT with literature (J.Am.Chem. Soc 2010, 132, 11437) can be compound 7 (named ID in this patent) in the attached information. The specific method is as follows:
[0077] Take 2.32 g (2.0 mmol) of 4,9-dihydro-4,4,9,9-tetrakis(hexadecyl)-s-inda[1,2-b:5,6-b'] -Dithiophene (ID) was placed in a 100mL two-necked bottle under nitrogen protection. Add 30 mL of dry tetrahydrofuran to dissolve. The reaction flask was placed in acetone liquid nitrogen at -78°C. When the temperature of the reaction system dropped to -78°C, 4.4 mmol of n-butyllithium was added dropwise, and the acetone bath was removed after...
Embodiment 2
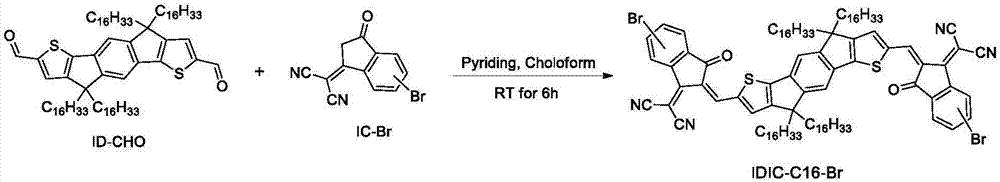
[0084] Example 2. Synthesis of polymer represented by formula IDIC-C16-Br
[0085] Its reaction equation is as figure 2 shown. Concrete reaction steps and reaction conditions are as follows:
[0086]
[0087]Take ID-CHO (0.9 g, 0.74 mmol) and IC-Br (1.0 g, 3.65 mmol, according to the literature Eur.J.Org.Chem.2016, 2016, 2404, Materials Chemistry Frontiers 2017, 1, 1389. Synthesized), This was dissolved in chloroform (50 mL), evacuated with argon for 5 minutes, 0.7 mL of pyridine was added, and the reaction was stopped after 8 hours at room temperature. The reaction solution was slowly precipitated into methanol (200 mL), the precipitated solid was filtered, rinsed with methanol, and vacuum-dried for one day to obtain 1.1 g of the compound represented by the formula IDIC-C16-Br as a powder, with a yield of 85.9% .
[0088] Structural corroboration data: 1 H NMR (400MHz, CDCl 3 ), δ(ppm): 8.98-9.02(m, 2H), 8.75-8.86(m, 1H), 7.78-8.58(m, 5H), 7.70-7.80(s, 2H), 7.76-7.6...
Embodiment 3
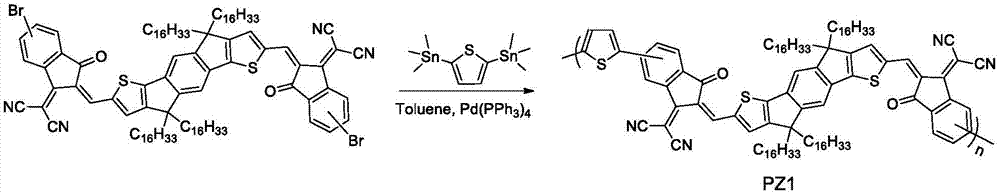
[0091] Embodiment 3, the synthesis of polymer represented by formula PZ1
[0092] Its reaction equation is as image 3 shown. Concrete reaction steps and reaction conditions are as follows:
[0093]
[0094] Referring to the literature (Chem.Mater., 2012, 24(16), 3247-3254), take 0.02 mmol each of monomer IDIC-C16-Br and M1, dissolve it in a mixed solvent of toluene (8 mL) and DMF (2 mL) After that, the air was evacuated with argon for 5 minutes, the catalyst tetrakis(triphenylphosphine)palladium(0) (10 mg) was added, and the air was continued to be evacuated for 25 minutes, and then the polymerization was stopped at the reflux temperature of toluene for 14 hours. The polymer solution was cooled to room temperature and slowly precipitated into methanol (50 mL). The precipitated solid polymer was eluted with methanol and n-hexane in sequence in a Soxhlet extractor. Finally, it was dissolved in chloroform, precipitated into methanol, filtered, and vacuum-dried for one day ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar absorptivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com