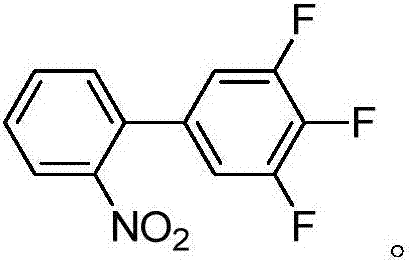
Preparation method of 3,4,5-trifluoro-2'-nitro-1,1'-biphenyl
A technology for nitrobiphenyl and nitrobenzoate is applied in the field of preparation of 3,4,5-trifluoro-2'-nitrobiphenyl, and can solve the problems of high cost, complicated steps and high risk. , to achieve the effect of low production cost, short synthesis steps and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In this embodiment, 3,4,5-trifluoro-2'-nitrobiphenyl is prepared by the following method, which specifically includes the following steps:
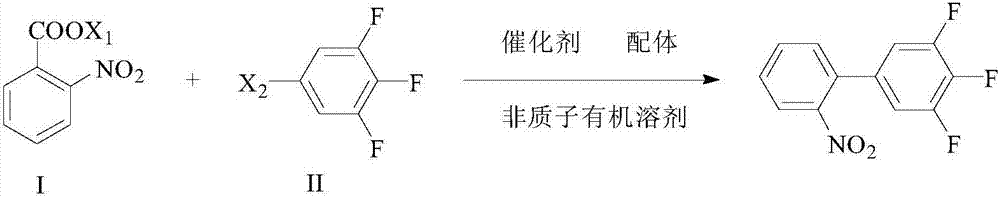
[0043] (1) Preparation of o-nitrobenzoate
[0044] Add 100g of o-nitrobenzoic acid, 470mL of absolute ethanol to a 1L three-necked flask, and add potassium hydroxide ethanol solution dropwise at room temperature [41.2g potassium hydroxide (85% by mass) dissolved in 470mL of absolute ethanol] After stirring for 2 hours at room temperature, a large amount of light yellow solid precipitated during this process, filtered and dried to obtain 116 g of light yellow powdery solid potassium salt of o-nitrobenzoic acid.
[0045] (2) Preparation of 3,4,5-trifluoro-2'-nitrobiphenyl
[0046] Add 108g o-nitrobenzoic acid potassium salt, 105.5g 3,4,5-trifluorobromobenzene, 2.1g triphenylphosphine, 0.6g 1,10-phenanthroline, 0.28g sodium chloride in a 2L three-necked flask Copper, 65mg bis(dibenzylideneacetone)palladium and 500g N,N-dimethylformamide. Re...
Embodiment 2
[0048] Add 109g o-nitrobenzoic acid sodium salt, 105.5g 3,4,5-trifluorobromobenzene, 2.4g 2-(di-tert-butylphosphine)biphenyl, 0.6g 1,10-phenanthroline into a 2L three-necked flask, 0.53g cuprous iodide, 0.7g Pd / C and 500g trimethylbenzene. Replace nitrogen for three times, stir and warm to 150°C, react for 30 hours, then cool to room temperature, filter, recover the palladium catalyst from the filter cake, and wash the filtrate twice with 1% (w / w) hydrochloric acid solution to separate the organic phase and remove After the trimethylbenzene was dissolved and recovered, the crude product was obtained, which was recrystallized with isobutanol to obtain 101 g of 3,4,5-trifluoro-2'-nitrobiphenyl with a yield of 80%. The content of the product was 96% by quantitative HPLC analysis.
Embodiment 3
[0050] Add 108g o-nitrobenzoic acid potassium salt, 83.3g 3,4,5-trifluorochlorobenzene, 2.1g triphenylphosphine, 0.6g 1,10-phenanthroline, 0.4g bromide into a 2L three-necked flask Copper, 0.7g MS-Pd and 500g trimethylbenzene. Replace the nitrogen for three times, stir and raise the temperature to 160° C., react for 20 hours, then cool to room temperature, filter, and recover the palladium catalyst from the filter cake. The filtrate was washed twice with 1% (w / w) hydrochloric acid solution, the organic phase was separated, and the crude product was obtained after desolvation and recovery of trimethylbenzene, which was recrystallized with isobutanol to obtain 114g of 3,4,5-trifluoro-2'-nitrate The yield of biphenyl was 90%, and the content of the product was 97% by quantitative HPLC analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com