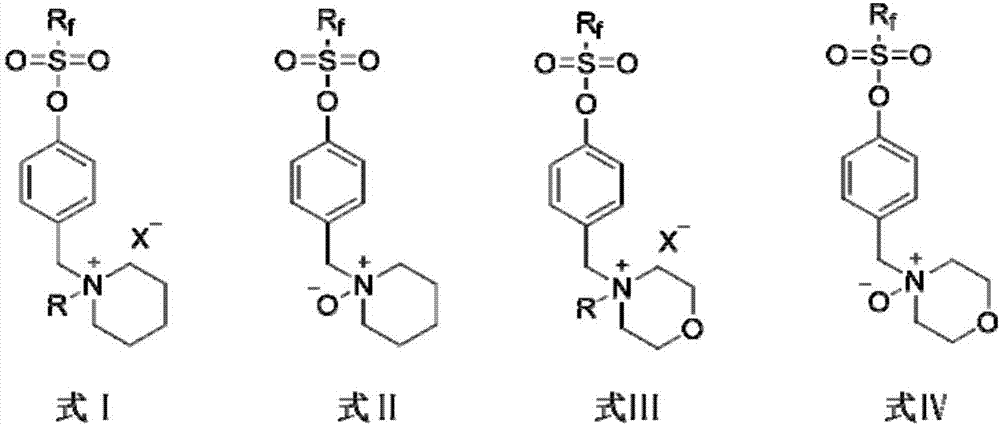
Oil-type fire-hazard fluorine-containing fire extinguishing agent, liquid hydrocarbon evaporation-inhibiting agent, and preparation method and applications thereof
A solvent and surfactant technology, which is applied in the fields of fluorine-containing fire extinguishing agents and steam suppressors for oil fires, and can solve problems such as long synthesis routes of fluorine-containing surfactants, low total yield of target products, and effects on fire fighting operations , to achieve high industrial application value, excellent surface activity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
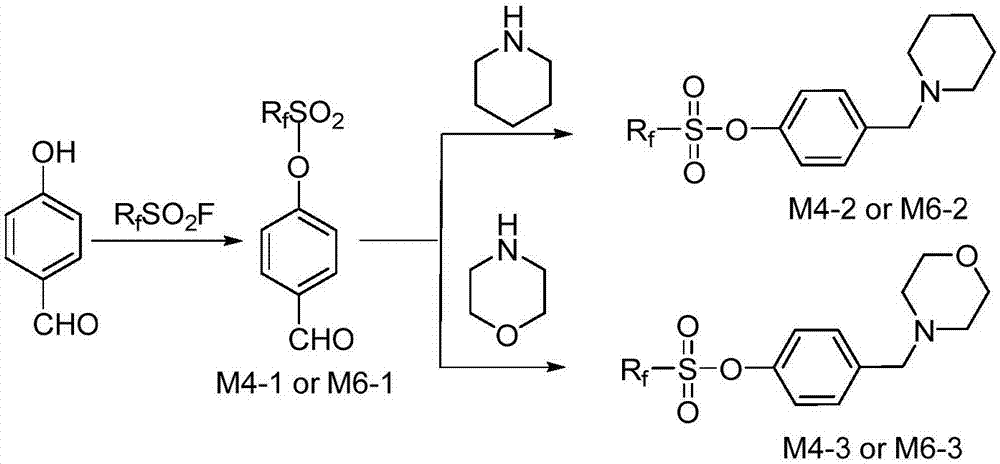
[0037] The preparation of embodiment 1 intermediate (M6-1 or M4-1)
[0038] Add 12.2g (0.1mol) of p-hydroxybenzaldehyde into a dry 250mL flask, dissolve it with 150mL of acetonitrile, add 27.6g (0.2mol) of acid-binding agent potassium carbonate under stirring, reflux for half an hour, and then slowly drop the fluorine-containing precursor The raw material is 48.2 g (0.12 mol) of perfluorohexylsulfonyl fluoride or 36.2 g (0.12 mol) of perfluorobutylsulfonyl fluoride, and the end point of the reaction is monitored by TLC. Add 100mL ethyl acetate to the reaction solution, wash 3 times with saturated sodium chloride solution, dry the organic layer with anhydrous sodium sulfate and crystallize at low temperature, filter to obtain the white solid intermediate perfluorohexylsulfonyloxybenzaldehyde (M6- 1) 47.9g (95% yield) or 39.2g (97% yield) of white solid intermediate perfluorobutylsulfonyloxybenzaldehyde (M4-1).
[0039]
[0040] 1 H NMR (400MHz, CDCl 3 ): δ10.05(s, 1H, -CH...
Embodiment 2
[0047] The preparation of embodiment 2 intermediate (M6-2 or M4-2)
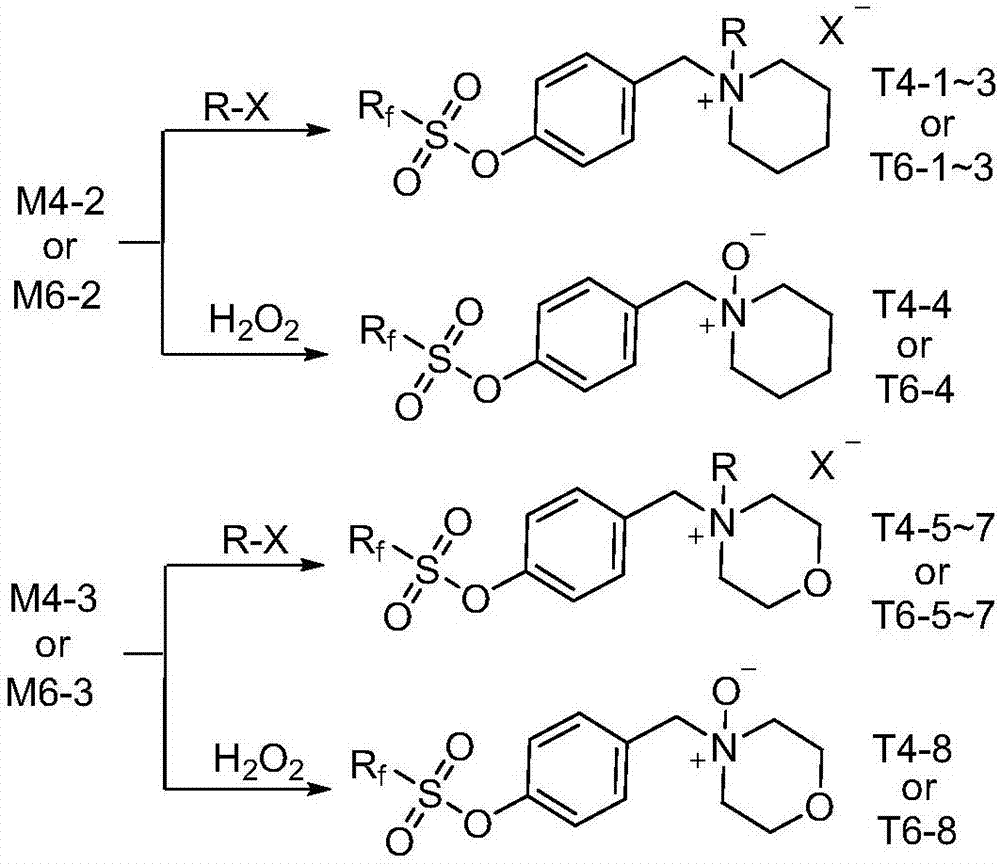
[0048] Add 10g (0.02mol) of intermediate M6-1 or 8g (0.02mol) of intermediate M4-1, 100mL of anhydrous methanol into a dry 250mL flask, then add 1.7g (0.02mol) of piperidine, and stir at room temperature for 2h. Then 0.76 g (0.02 mol) of sodium borohydride was added in batches, and the reaction was continued for 10 min, and the end point of the reaction was monitored by TLC. 100mL ethyl acetate was added to the reaction solution, washed three times with saturated sodium chloride solution, the organic layer was dried over anhydrous sodium sulfate and crystallized at low temperature to obtain the white solid intermediate perfluorohexylsulfonyloxybenzylpiperidine (M6- 2) 10.8g (94% yield) or 8.8g (93% yield) of the colorless liquid intermediate perfluorobutylsulfonyloxybenzylpiperidine (M4-2).
[0049]
[0050] 1 H NMR (400MHz, CDCl 3 ): δ7.40(d, J=8.0Hz, 2H, phH), 7.21(d, J=8.0Hz, 2H, phH), 3.46(s, 2H, ph...
Embodiment 3
[0057] The preparation of embodiment 3 intermediate (M6-3 or M4-3)
[0058] Add 10g (0.02mol) of intermediate M6-1 or 8g (0.02mol) of intermediate M4-1, 100mL of anhydrous methanol into a dry 250mL flask, add 1.74g (0.02mol) of morpholine, and stir at room temperature for 2h. Then 0.76 g (0.02 mol) of sodium borohydride was added in batches, and the reaction was continued for 10 min, and the end point of the reaction was monitored by TLC. 100mL ethyl acetate was added to the reaction solution, washed three times with saturated sodium chloride solution, the organic layer was dried over anhydrous sodium sulfate and crystallized at low temperature to obtain the white solid intermediate perfluorohexylsulfonyloxybenzylmorpholine (M6- 3) 11.0 g (96% yield) or 8.7 g (92% yield) of white solid intermediate perfluorobutylsulfonyloxybenzylmorpholine (M4-3).
[0059]
[0060] 1 H NMR (400MHz, CDCl 3 ): δ7.43(d, J=8.0Hz, 2H, phH), 7.23(d, J=8.0Hz, 2H, phH), 3.72(t, J=4.0Hz, 4H, -CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com