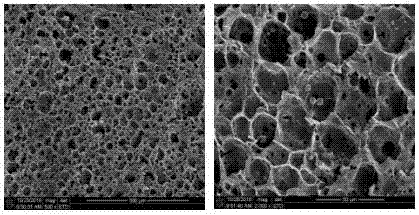
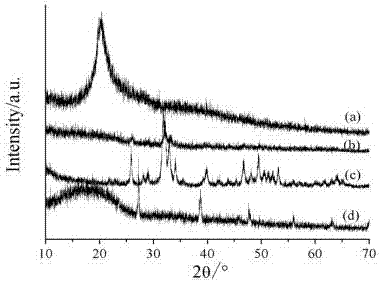
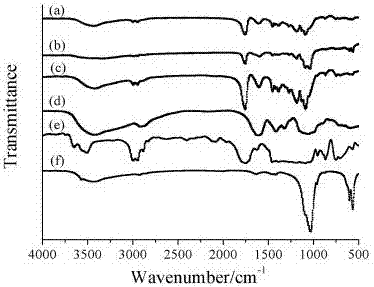
Nano-hydroxyapatite/carboxymethyl chitosan/poly(lactic-co-glycolic acid) micro-nano hybrid drug-loaded scaffold and bionic preparation method thereof
A nano-hydroxyapatite, carboxymethyl chitosan technology, applied in medical science, tissue regeneration, prosthesis, etc., can solve the problem of poor hydrophilicity and biocompatibility, lack of cell recognizable sites, lack of Biological activity and other issues, to achieve the effect of improving biological activity, good biocompatibility, and achieving complementary functional advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1) Disperse 0.075g of nano-hydroxyapatite in 20mL of deionized water, and sonicate for 10min to make it evenly dispersed;
[0041] 2) Add 0.2g carboxymethyl chitosan to it, and stir with a magnetic stirrer for 1h to form a uniform water phase (continuous phase);
[0042] 3) Take 136 µg icariin and add it to the above aqueous phase to prepare a molar concentration of 10 -5 M's icariin solution;
[0043] 4) Dissolve 0.32g of polylactic glycolic acid in 8mL of dichloromethane to prepare the oil phase (dispersed phase) of the emulsion;
[0044] 5) Take 5mL of the emulsion water phase and 2mL of the oil phase, add them to the mold, and add 14.5 µL of glutaraldehyde to it with the molar ratio of amino groups and aldehyde groups at 1:1, and use the Ultra Turrax T25 high-speed emulsifier at 15×10 3 Emulsify at rpm / min, emulsification time is 1min;
[0045] 6) Place the emulsion at room temperature for 12 hours to evaporate the solvent, then transfer it to -10~-80°C and free...
Embodiment 2
[0047] 1) Disperse 0.15g of nano-hydroxyapatite in 20mL of deionized water, and sonicate for 10min to make it evenly dispersed;
[0048] 2) Add 0.2g carboxymethyl chitosan to it, and stir with a magnetic stirrer for 1h to form a uniform water phase (continuous phase);
[0049] 3) Take 136µg icariin and add it to the above aqueous phase to prepare a molar concentration of 10 -5 M's icariin solution;
[0050] 4) Dissolve 0.32g of polylactic-glycolic acid in 8mL of dichloromethane to prepare the oil phase (dispersed phase) of the emulsion;
[0051] 5) Take 5mL of the emulsion water phase and 2mL of the oil phase, put them into the mold, and add 14.5 µL of glutaraldehyde to it with the molar ratio of amino group and aldehyde group at 1:1, and use the Ultra Turrax T25 high-speed emulsifier at 15×10 3 Emulsify at rpm / min, emulsification time is 1min;
[0052] 6) Place the emulsion at room temperature for 12 hours to evaporate the solvent, then transfer it to -10~-80°C and freeze...
Embodiment 3
[0054] 1) Disperse 0.3g of nano-hydroxyapatite in 20mL of deionized water, and sonicate for 10min to make it evenly dispersed;
[0055] 2) Add 0.2g carboxymethyl chitosan to it, and stir with a magnetic stirrer for 1h to form a uniform water phase (continuous phase);
[0056] 3) Take 136µg icariin and add it to the above aqueous phase to prepare a molar concentration of 10 -5 M's icariin solution;
[0057] 4) Dissolve 0.32g of polylactic-glycolic acid in 8mL of dichloromethane to prepare the oil phase (dispersed phase) of the emulsion;
[0058] 5) Take 5mL of the emulsion water phase and 2mL of the oil phase, put them into the mold, and add 14.5 µL of glutaraldehyde to it with the molar ratio of amino group and aldehyde group at 1:1, and use the Ultra Turrax T25 high-speed emulsifier at 15×10 3 Emulsify at rpm / min, emulsification time is 1min;
[0059] 6) Place the emulsion at room temperature for 12 hours to evaporate the solvent, then transfer it to -10~-80°C and freeze ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com