Special clinical nutrition formula for climacterium women, and preparation method of special clinical nutrition formula
A nutritional formula and special-purpose technology, which is applied in the field of clinical nutritional formula and its preparation for menopausal women, can solve the problems of syndrome differentiation and classification of menopausal syndrome, lack of unified standards for curative effect evaluation, complicated clinical prescriptions, scattered research work, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
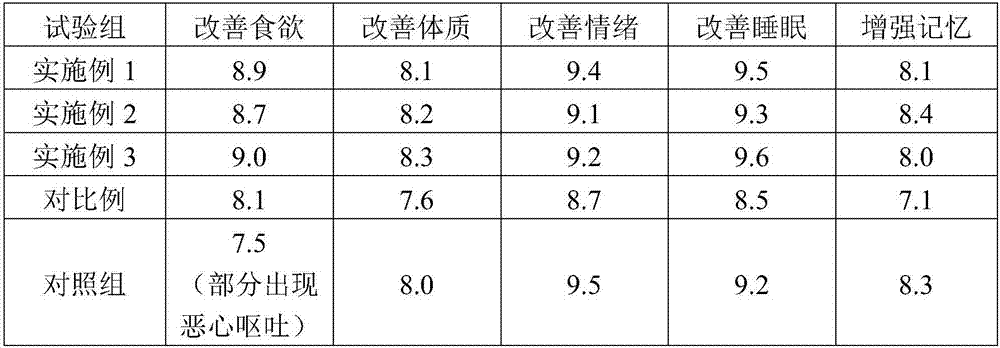
Embodiment 1
[0071] The climacteric women-specific clinical nutritional formula of the present invention includes 10.33g of whey protein powder, 5.17g of soybean protein isolate, 12g of whole milk powder, 0.33g of bovine colostrum powder, 0.17g of hydrolyzed fish collagen, and 0.12g of taurine ; olive oil 2.64g, soybean oil 4.26g, coconut oil 2.64g, perilla oil 0.6g, medium chain triglyceride 0.2g; maltodextrin 34.49g, tapioca starch 4.94g, rock sugar 6.3g; water-soluble dietary fiber ( Including galacto-oligosaccharides) 3.5g, soybean fiber 1g; macro elements calcium 0.43g, phosphorus 0.236g, potassium 0.093g, sodium 0.248g, magnesium 0.148g; trace elements iron 0.011g, zinc 0.008g; vitamin A 0.0005g , β-carotene 0.017g, vitamin D 3 0.000001g, Vitamin E 0.009g; Vitamin B 1 0.001g, Vitamin B 2 0.001g, Vitamin B 6 0.001g, Vitamin B 12 0.0000013g, vitamin C 0.104g, pantothenic acid 0.003g, folic acid 0.00023g, niacin 0.0133g; soybean powder 1.67g, orange red 0.17g, seabuckthorn 0.17...
Embodiment 2
[0073] The climacteric women-specific clinical nutritional formula of the present invention includes 10.33g of whey protein powder, 5.17g of soybean protein isolate, 12g of whole milk powder, 0.33g of bovine colostrum powder, 0.07g of hydrolyzed fish collagen, and 0.12g of taurine ; olive oil 2.64g, soybean oil 4.26g, coconut oil 2.64g, perilla oil 0.6g, medium chain triglyceride 0.2g; maltodextrin 34.49g, tapioca starch 4.94g, rock sugar 6.3g; water-soluble dietary fiber ( Including galacto-oligosaccharides) 3.5g, soybean fiber 1g; macro elements calcium 0.43g, phosphorus 0.236g, potassium 0.093g, sodium 0.248g, magnesium 0.148g; trace elements iron 0.011g, zinc 0.008g; vitamin A 0.0005g , β-carotene 0.017g, vitamin D 3 0.000001g, Vitamin E 0.009g; Vitamin B 1 0.001g, vitamin B2 0.001g, vitamin B 6 0.001g, Vitamin B 12 0.0000013g, Vitamin C 0.104g, pantothenic acid 0.003g, folic acid 0.00003g, niacin 0.0133g; Skin 0.17g, kudzu root 0.2g, L-arabinose 0.01g, γ-aminobuty...
Embodiment 3
[0075] The climacteric women-specific clinical nutritional formula of the present invention includes 10.33g of whey protein powder, 5.17g of soybean protein isolate, 12g of whole milk powder, 0.33g of bovine colostrum powder, 0.07g of hydrolyzed fish collagen, and 0.12g of taurine ; olive oil 2.64g, soybean oil 4.26g, coconut oil 2.64g, perilla oil 0.6g, medium chain triglyceride 0.2g; maltodextrin 34.49g, tapioca starch 4.94g, rock sugar 6.3g; water-soluble dietary fiber ( Including galacto-oligosaccharides) 3.5g, soybean fiber 1g; macro elements calcium 0.43g, phosphorus 0.236g, potassium 0.093g, sodium 0.248g, magnesium 0.148g; trace elements iron 0.011g, zinc 0.008g; vitamin A 0.0005g , β-carotene 0.017g, vitamin D 3 0.000001g, Vitamin E 0.009g; Vitamin B 1 0.001g, Vitamin B 2 0.001g, Vitamin B 6 0.001g, Vitamin B 12 0.0000013g, Vitamin C 0.104g, pantothenic acid 0.003g, folic acid 0.00003g, niacin 0.0133g; Skin 0.07g, kudzu root 0.02g, L-arabinose 0.01g, γ-amino...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com