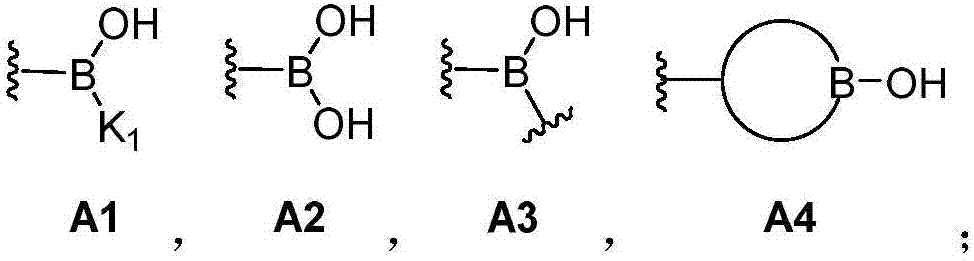
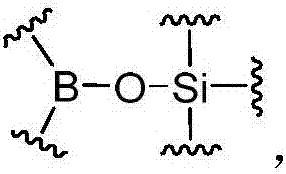Dynamic polymer with dynamic cross-linked structure
A dynamic cross-linking and polymer technology, applied in the field of intelligent polymers, can solve the problems of poor stability of mercaptans, poor dynamic activity of ester bonds, slowness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0261] In the above-mentioned preparation embodiment, an appropriate amount of monofunctional organoboron compound (I) and / or monofunctional silicon-containing compound (II) components can also be selectively introduced, which can be adjusted through component formulation , to obtain the dynamic cross-linked structure. Monofunctional compounds can play a role in adjusting crosslink density, dynamics, mechanical strength and so on.
[0262] In the preparation embodiment described above, the reaction of other reactive groups can also be achieved by introducing organic boronic acid groups and / or organic boronic acid ester groups, silicon hydroxyl and / or silicon hydroxyl precursors, organic boric acid silicon ester bonds However, compound components containing other reactive groups can be achieved together. The compound containing only other reactive groups can be any suitable compound that can achieve the reaction with other reactive groups in organoboron compound (I) and / or sil...
Embodiment 1
[0412] A dynamic polymer with a dynamic cross-linking structure is prepared by using a small-molecule organoboron compound (I) containing four functional groups and a small-molecule silicon-containing compound (II) containing two functional groups.
[0413]
[0414] Weigh a certain amount of organoboron compound (a) (using AIBN as the initiator and triethylamine as the catalyst, using vinylboronic acid, vinylboronic acid dibutyl ester and 1,6-hexanedithiol through thiol-ene click reaction Prepared) dissolved in tetrahydrofuran solvent to prepare a 0.8mol / L solution; Measure 40ml of tetrahydrofuran solution dissolved with organoboron compound and pour it into a dry and clean flask, add 4ml of deionized water, add dropwise a little acetic acid and mix evenly, Slowly add 5.02g of silicon-containing compound (b) therein again (using dimethylallyl chlorosilane, 1,10-decanedithiol as raw material, taking AIBN as initiator and triethylamine as catalyst, through thiol-ene click rea...
Embodiment 2
[0416] A dynamic polymer with a dynamic cross-linking structure is prepared by using a small-molecule organoboron compound (I) containing four functional groups and a macromolecular silicon-containing compound (II) containing two functional groups.
[0417]
[0418] Add 15ml of phenylboronic acid-terminated polyethylene glycol (a) into a dry and clean three-necked flask (with polyethylene glycol 400 and 2-bromopropionyl bromide as raw materials and triethylamine as a catalyst to prepare dibromo-terminated polyethylene glycol (a) diol, and then it and 2-aminomethylphenylboronic acid are obtained through alkylation reaction to obtain the final product), heated to 80°C, in which a small amount of deionized water and acetic acid are added dropwise, and then 42ml of acetic acid is added dropwise under stirring The methoxysilane-modified silicone oil (b) (made from dimethylvinylmethoxysilane and hydrogen-terminated silicone oil with a viscosity of about 2000mPa·s as raw materials,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| shear viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com