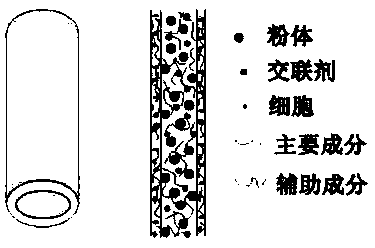
Fibers for tissue engineering bone repairing, tissue engineering bone repairing scaffold and preparation method thereof
A tissue-engineered bone and fiber technology, applied in medical science, prosthesis, etc., can solve the problems of high melting temperature, biochemical damage, enhanced physical damage, etc., achieve good mechanical properties, excellent and rapid osteogenesis and angiogenesis, The effect of maintaining cell viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] In this example, agarose with a low gelation temperature is used as the main component of the hydrogel preparation material, and fibrin is used as an auxiliary component of the hydrogel preparation material. The powder material is self-made 10-50 μm bioglass powder, and the growth factor is selected. Platelet-derived growth factor (PDGF), the printed cells are bone marrow mesenchymal stem cells. The specific implementation steps are as follows:
[0050] (1) The 58S bioglass was prepared by the sol-gel method, that is, a certain amount of tetraethyl orthosilicate, triethyl phosphate, and calcium nitrate were dissolved in deionized water, and after standing at room temperature for 24 hours, the glass gel was taken out and Vacuum dried, after drying at 650 o Heat treatment at C for 5 hours, ball milling, sieving, and irradiation sterilization to obtain sterile biological glass powder with the required particle size.
[0051] (2) Put sterile agarose at 90 o Dissolve in n...
Embodiment 2
[0056] In this example, sodium alginate is used as the main component of the hydrogel preparation material, and hyaluronic acid is used as the auxiliary component of the hydrogel preparation material. The powder material is self-made polycaprolactone microspheres of 20-100 μm, and the growth factor is bone morphology. BMP-2, the printed cells are osteoblasts. The specific implementation steps are as follows:
[0057] (1) Polycaprolactone microspheres were prepared by solvent evaporation method. That is, polycaprolactone with a molecular weight of 50000 is dissolved in dichloromethane, and the concentration of the prepared solution is 5 wt%. Dissolve 1799-type polyethylene glycol (PVA) in deionized water under heating conditions to obtain a PVA solution with a concentration of 1 wt%. Under the condition of stirring at a stirring rate of 800 rpm, the polycaprolactone solution was slowly added into the PVA solution with a needle, wherein the volume ratio of the polycaprolactone...
Embodiment 3
[0063] In this example, gelatin is used as the main component of the hydrogel preparation material, and polypeptide is the auxiliary component of the hydrogel preparation material. The powder material is selected from commercially available nano-hydroxyapatite powder of 20-100 nm, and the growth factor is selected from fibroblast growth. Factor (FGF), the printed cells are selected from adipose stem cells. The specific implementation steps are as follows:
[0064] (1) Nano-hydroxyapatite was purchased from Sigma in the United States and sterilized by irradiation before use. Mix sterile gelatin and peptide at 37 o Dissolve in sterile phosphate buffer at C to make the first sol of 15wt% gelatin+5wt% polypeptide and the second sol of 10wt% gelatin+5wt% polypeptide. Add nano-hydroxyapatite powder into the first sol and mix evenly, the powder addition amount is 20wt%, to obtain sol-powder composite viscous slurry.
[0065] (2) Add a certain amount of transglutaminase and fibrobl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com